It’s a familiar, sinking feeling. You reach into the cabinet for a critical sample or process chemical, only to find the container has subtly failed. Perhaps the bottle is slightly warped, the lid feels brittle, or you see a faint cloudiness in a solution that should be crystal clear.
The immediate result is a lost sample and a wasted afternoon. But the true cost runs much deeper. That single point of failure triggers a cascade of questions: Is the rest of the batch compromised? Can we trust the data from our last analysis? How much will this delay the project?
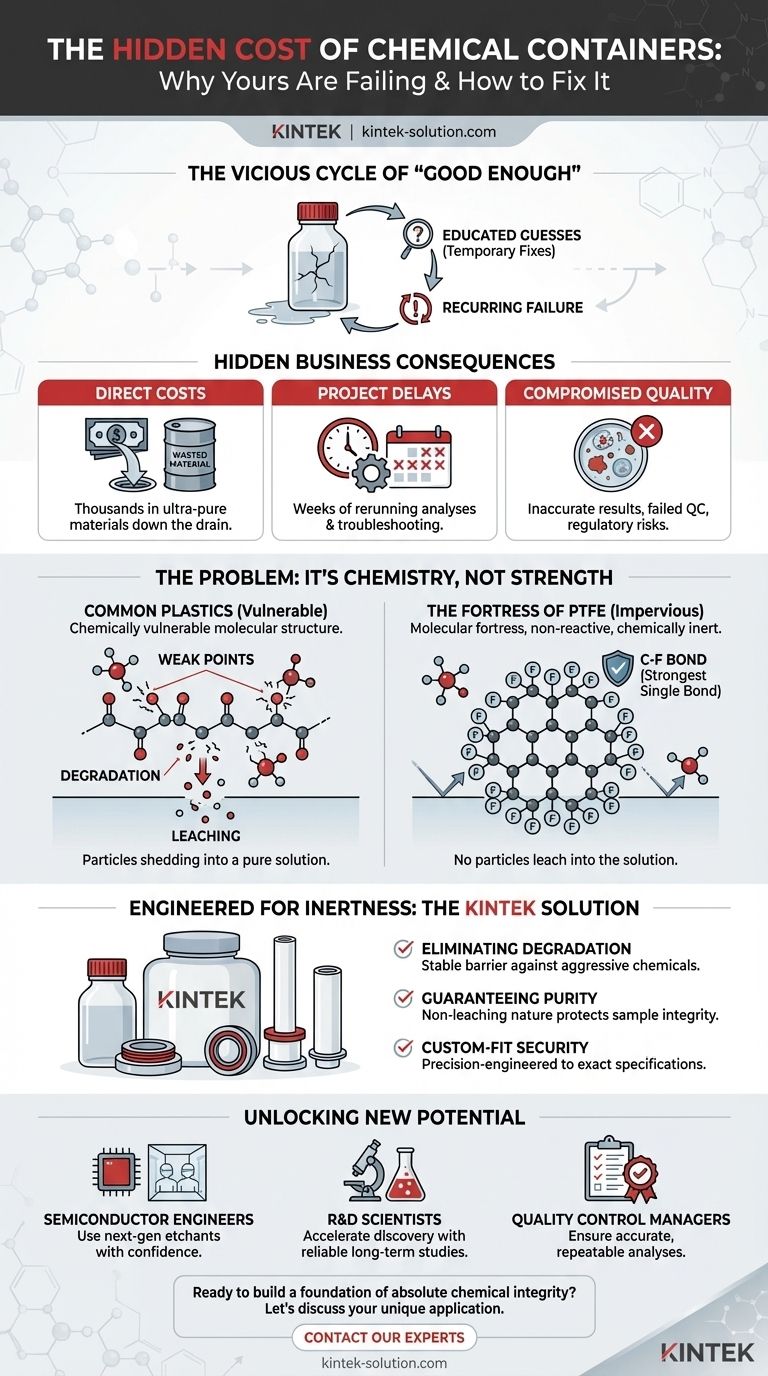
You’re not alone. In labs and high-tech manufacturing facilities worldwide, teams are caught in a frustrating cycle of trying to outsmart chemical compatibility.
The Vicious Cycle of "Good Enough" Solutions
When a container fails, the typical response is a series of educated guesses. Maybe a thicker-walled HDPE bottle will work? Or perhaps we switch from polypropylene to another specialty polymer that the compatibility chart claims is "excellent"?
These are logical steps, but they are often temporary fixes. You might get a few more weeks or months of stability, but you haven't solved the underlying problem. You're just postponing the next failure.
The business consequences of this cycle are severe and often hidden:
- Direct Costs: A single liter of ultra-pure semiconductor-grade acid can cost hundreds or even thousands of dollars. A compromised batch means that investment is literally down the drain.
- Project Delays: Rerunning analyses, re-qualifying materials, and troubleshooting contamination can add weeks or months to a development timeline, jeopardizing launch dates and market advantage.
- Compromised Quality: In medical and pharmaceutical settings, even trace amounts of leached plasticizers from a container can contaminate reagents, leading to inaccurate diagnostic results or failed quality control.
The struggle isn't a result of carelessness; it's the result of looking at the problem from the wrong angle. The solution isn't about finding a stronger plastic, but a fundamentally different one.
The Problem Isn't Strength, It's Chemistry
Most plastics fail not because they lack physical strength, but because they are chemically vulnerable at a molecular level. Think of their polymer chains as having tiny "handles" or weak points. Aggressive chemicals—strong acids, bases, or organic solvents—latch onto these handles and begin to tear the material apart. This is degradation. At the same time, the plastic itself can shed molecules (leaching) into your pure solution.
This is why thicker walls or different grades of common plastics often fail. They are just bigger or slightly modified versions of the same vulnerable structure. They might resist attack for longer, but the fundamental weakness remains.
The Fortress of PTFE
This is where Polytetrafluoroethylene (PTFE) changes the game. Its legendary chemical resistance isn't magic; it's a direct result of its unique molecular architecture.
The core of PTFE is a long chain of carbon atoms, but each carbon atom is completely shielded by a barrier of fluorine atoms. The carbon-fluorine (C-F) bond is one of the strongest single bonds in all of organic chemistry.
A simplified view of the PTFE molecule. The fluorine atoms form an impenetrable, non-reactive shield around the carbon backbone.
This creates a molecular fortress. There are no "handles" for chemicals to grab. The surface is incredibly stable, non-reactive, and smooth. Aggressive chemicals have nowhere to initiate an attack, and the material has nothing to leach into your solution. It is, for nearly all practical purposes, chemically inert.
The rare exceptions—like molten alkali metals or high-pressure fluorine gas—are so extreme they prove the rule. For the vast majority of acids, bases, solvents, and reactive agents used in labs and industry, PTFE offers complete and permanent security.
Engineered for Inertness: The Right Tool for the Job
To truly solve the problem of container failure and contamination, you need to stop treating the symptoms and address the root cause. You need a tool designed with a deep understanding of this molecular reality.
This is the principle that guides KINTEK's manufacturing. We create precision-engineered PTFE components—from labware and bottles to custom seals and liners—that are the physical embodiment of this chemical inertness.
Our products are not just "resistant"; they are designed to be impervious.
- Eliminating Degradation: By leveraging the strength of the carbon-fluorine bond, our PTFE components provide a stable, reliable barrier against the most aggressive chemicals, ensuring the integrity of your process.
- Guaranteeing Purity: The non-leaching nature of PTFE means your high-purity samples, reagents, and process fluids remain completely uncontaminated, protecting the validity of your results and the quality of your final product.
- Custom-Fit Security: Whether you need a standard bottle for acid storage or a complex, custom-machined liner for a semiconductor processing tank, we fabricate components to your exact specifications. This ensures a perfect fit for your application, eliminating another potential point of failure.
Choosing KINTEK's PTFE components isn't just about buying a better container. It's about making a strategic decision to eliminate a fundamental variable from your critical processes.
Beyond the Fix: Unlocking New Potential
When you no longer have to second-guess the integrity of your containers, you unlock new levels of efficiency and innovation. The mental energy once spent on risk management can now be focused on pushing boundaries.
- For Semiconductor Engineers: You can confidently use next-generation, highly aggressive etchants and cleaning agents, knowing your process fluids will remain ultra-pure and your equipment is protected.
- For R&D Scientists: You can run long-term stability studies on reactive compounds without the container itself becoming a variable, accelerating the discovery and validation of new materials and medicines.
- For Quality Control Managers: You can trust that your reference standards and reagents are pristine, ensuring every analysis is accurate and repeatable, month after month.
Ultimately, solving the chemical containment problem moves your team from a reactive state of managing failures to a proactive state of enabling innovation.
Your work is too critical to be compromised by a simple plastic container. If you're ready to move beyond "good enough" and build a foundation of absolute chemical integrity for your projects, our team of specialists is here to help you design the perfect solution. Let's discuss the unique demands of your application. Contact Our Experts.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
Related Articles
- The Unseen Workhorse: Why PTFE Is the Default Choice for Impossible Problems
- The Unseen Component: How PTFE Became the Bedrock of Medical Device Reliability
- The Physics of a Perfect Fit: How PTFE Eliminates an Athlete's Hidden Distractions
- How PTFE Solves Critical Industrial Challenges Through Material Superiority
- Why Your High-Performance PTFE Parts Fail—And Why It's Not the Material's Fault




















