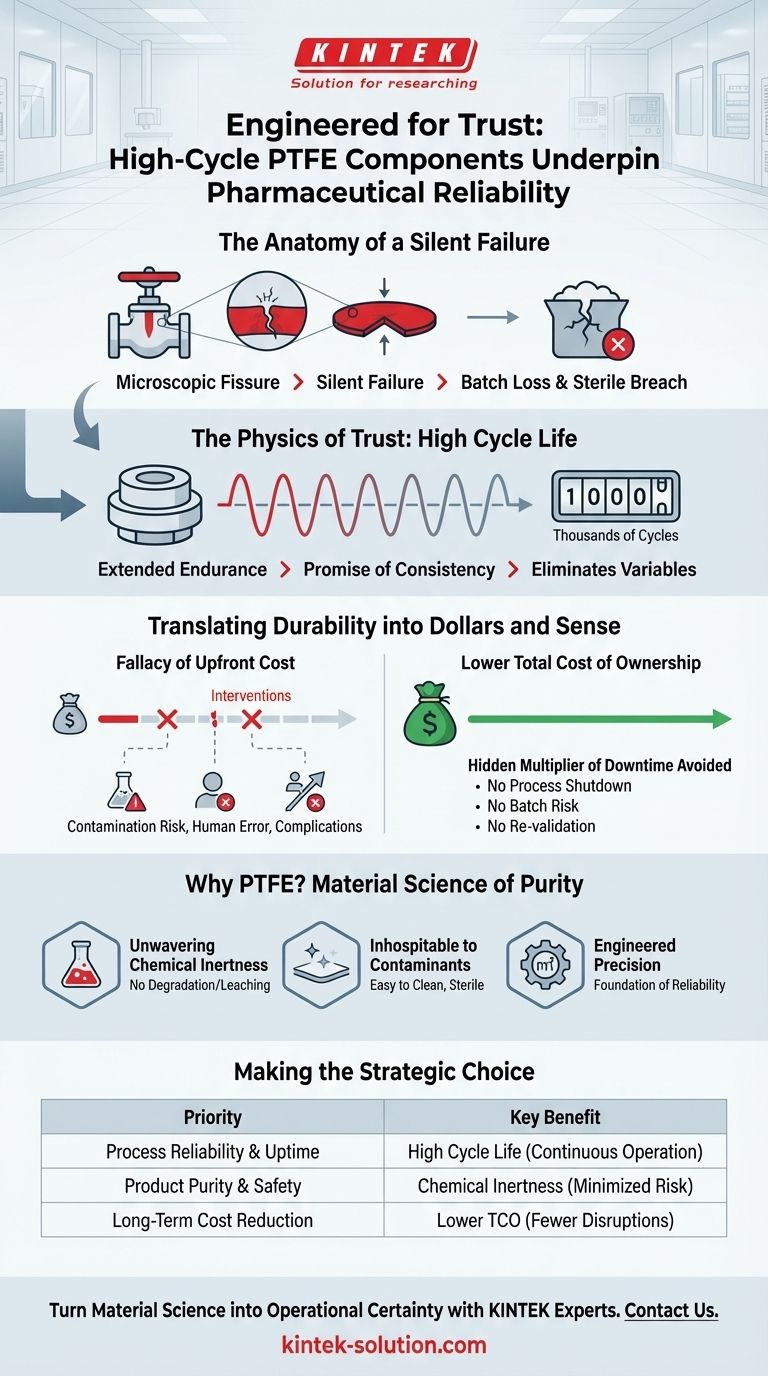
The Anatomy of a Silent Failure
Imagine a validated, sterile production line running a multi-million dollar batch of a life-saving biologic. Every sensor is green, every pressure is nominal. The system is a fortress of control.
But deep inside a critical valve, an unseen process is underway. After thousands of cycles, a microscopic fissure forms in its diaphragm seal. It’s a tiny imperfection, invisible and inconsequential—until it’s not.
This is the anatomy of a silent failure. It isn't a sudden, catastrophic event. It's a slow erosion of trust in a system designed for perfection. The true cost isn't measured in a single failed component, but in the potential loss of the entire batch, the expensive ordeal of re-validation, and the breach of the sterile barrier that protects patient safety.
The Physics of Trust: What is Cycle Life?
A component's "cycle life" is the engineering term for its endurance. It’s the number of times a valve can open and close, a seal can compress and release, or a liner can withstand a process fluid before its performance degrades.
In pharmaceutical manufacturing, cycle life is more than a number on a spec sheet. It's a promise of consistency.
A high cycle life means the component—the PTFE diaphragm at the heart of the valve—remains perfectly functional for a much longer operational period. This isn't about avoiding the inconvenience of a replacement; it's about eliminating a variable in an equation where precision is paramount.
Translating Durability into Dollars and Sense
The decision to invest in high-performance components is often framed around cost. This is a cognitive trap. The conversation shouldn't be about the purchase price, but about the total cost of ownership—a calculation dominated by the catastrophic expense of failure.
The Fallacy of Upfront Cost
A standard component may seem economical at first glance. But its lower cycle life introduces a predictable need for intervention. Each maintenance event is a planned disruption and, more importantly, a risk. It’s an opportunity for contamination, human error, or an unexpected complication.
High-cycle PTFE components fundamentally change this equation. Their longevity pushes maintenance intervals so far into the future that they become a negligible factor in operational planning.
The Hidden Multiplier of Downtime
In a validated pharmaceutical environment, downtime is never simple. An unexpected valve failure triggers a cascade:
- Process Shutdown: The entire line may halt.
- Batch Risk: The in-process batch may need to be quarantined or discarded.
- Investigation: A root-cause analysis is often required.
- Re-validation: In a worst-case scenario, parts of the process may need to be re-validated, a time-consuming and expensive undertaking.
The reliability offered by high-cycle components directly prevents these costly disruptions, turning a potential liability into a predictable asset.
Why PTFE? The Material Science of Purity
The exceptional cycle life of a PTFE component is powerful, but it becomes indispensable when combined with the material's inherent properties. This combination is why PTFE is a cornerstone of modern pharmaceutical equipment.
-
Unwavering Chemical Inertness: PTFE is famously non-reactive. It will not degrade when exposed to aggressive cleaning agents (CIP/SIP) or react with high-purity active pharmaceutical ingredients. It is a silent guardian, ensuring nothing leaches into the product.
-
Inhospitable to Contaminants: The surface of high-quality PTFE is remarkably smooth and non-porous. This slick surface inhibits microbial adhesion and biofilm formation, making it easy to clean and essential for maintaining sterility.
-
Engineered Precision: Achieving this level of material integrity isn't accidental. It requires obsessive precision in manufacturing. At KINTEK, we specialize in fabricating custom PTFE components—from seals and liners to labware—where every micron of surface finish and material purity counts. We know it's the foundation of a sterile and reliable process.
A System is More Than the Sum of its Parts
Even the most robust component can be compromised by a poorly designed system. A high-cycle PTFE diaphragm is a critical enabler of reliability, not a cure-all for systemic flaws.
Its benefits are only fully realized when:
- Installation is Precise: The valve must be correctly specified for the process pressures and temperatures. Automated actuation must be calibrated to avoid undue stress.
- System Design is Holistic: The component must be integrated into a system designed from the ground up for purity and efficiency, accounting for flow dynamics, piping layouts, and pumping pressures.
A superior component doesn't fix a bad system, but it elevates a good system to an exceptional one.
Making the Strategic Choice
Integrating high-cycle PTFE components is a strategic decision that aligns with core operational goals. The choice becomes clear when you define your priority.
| If Your Primary Focus Is... | Then the Key Benefit Is... |
|---|---|
| Process Reliability & Uptime | High Cycle Life, which ensures validated processes run without interruption from frequent valve maintenance. |
| Product Purity & Safety | Chemical Inertness, which minimizes contamination risks from both material leaching and maintenance interventions. |
| Long-Term Cost Reduction | Lower Total Cost of Ownership, derived from fewer replacements, less maintenance, and zero lost batches. |
Ultimately, choosing these components is a foundational step toward building a more robust, safe, and efficient manufacturing operation. Our expertise lies in turning material science into operational certainty. If your process demands uncompromising reliability and purity, Contact Our Experts.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Microwave Digestion Vessels for Demanding Applications
- Custom PTFE Teflon Parts Manufacturer Adjustable Height Flower Basket
Related Articles
- The Unseen Component: How PTFE Became the Bedrock of Medical Device Reliability
- When 'Chemically Inert' Isn't Enough: Why Your PTFE Components Fail and How to Prevent It
- Why Your High-Performance PTFE Parts Fail—And Why It's Not the Material's Fault
- The Physics of a Perfect Fit: How PTFE Eliminates an Athlete's Hidden Distractions
- The Asymmetric Cost of Failure: Why Precision PTFE Is Your Last Line of Defense




















