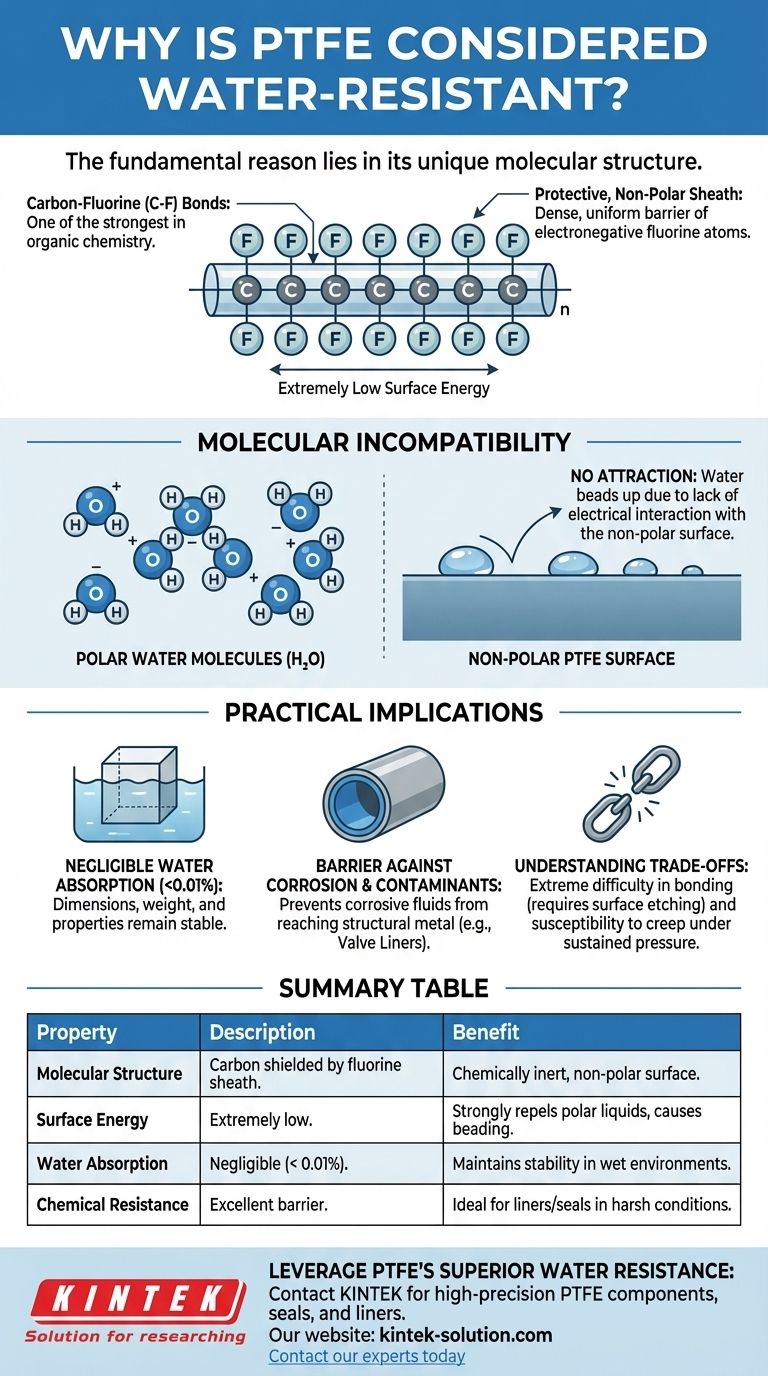
The fundamental reason PTFE is water-resistant lies in its unique molecular structure. Dominated by exceptionally strong carbon-fluorine bonds, the polymer chain is encased in a dense, uniform sheath of fluorine atoms. This configuration creates a chemically stable and non-polar surface with very low energy, which strongly repels polar liquids like water.
The core principle is molecular incompatibility. The highly electronegative fluorine atoms in PTFE create a stable, low-energy, non-polar surface that offers no attraction to the polar molecules of water, forcing the water to bead up and run off rather than wet or absorb into the material.

The Molecular Basis of PTFE's Water Resistance
To truly understand why PTFE (polytetrafluoroethylene) excels in wet environments, we must look at its chemistry at the atomic level. The properties are not accidental; they are a direct result of its composition.
The Dominance of the Carbon-Fluorine Bond
PTFE consists of a long chain of carbon atoms, but each carbon is bonded to two fluorine atoms. The carbon-fluorine (C-F) bond is one of the strongest single bonds in organic chemistry.
Fluorine is the most electronegative element, meaning it has an incredibly strong attraction for electrons. This creates a very stable, non-reactive bond that is difficult to break.
A Protective, Non-Polar "Sheath"
The fluorine atoms are larger than the carbon atoms they are attached to. They effectively wrap around the carbon backbone, creating a tight, uniform, and seamless protective sheath.
This sheath presents a surface that is electrically uniform and non-polar. There are no significant positive or negative charges for other molecules to latch onto.
The Critical Role of Low Surface Energy
The stable, non-polar sheath gives PTFE an extremely low surface energy. Think of surface energy as the level of attraction a material's surface has for other substances.
Materials with high surface energy (like clean metal) are easily "wetted" by liquids. Materials with low surface energy, like PTFE, are non-stick and repel liquids because there is minimal attractive force between the surface and the liquid.
Why Water Specifically is Repelled
Water (H₂O) is a polar molecule, with a slight positive charge on the hydrogen side and a slight negative charge on the oxygen side. These charges cause water molecules to stick to each other and to other polar surfaces.
When polar water comes into contact with the non-polar PTFE surface, there is no electrical attraction. The water molecules are more attracted to each other than to the PTFE, causing them to bead up into droplets with a high contact angle, minimizing their contact with the surface.
Practical Implications of This Property
This molecular structure translates directly into tangible benefits for real-world applications. It's not just about repelling surface water; it's about complete material integrity.
Negligible Water Absorption
Because there is no mechanism for water to bond with or seep into the material, PTFE exhibits almost zero moisture absorption (typically less than 0.01%).
This means its dimensions, weight, electrical insulating properties, and mechanical characteristics remain exceptionally stable even when fully submerged for long periods.
Barrier Against Corrosion and Contaminants
PTFE's water resistance also makes it a premier barrier material. It is frequently used as a liner or seat in valves and pipes, as mentioned in the butterfly valve example.
The PTFE lining prevents corrosive or reactive fluids dissolved in water from ever reaching the structural metal of the valve body, ensuring system longevity and purity.
Understanding the Trade-offs
While its water resistance is exceptional, the very properties that provide this benefit also create limitations that are critical to understand.
Extreme Difficulty in Bonding
The same low-energy, non-stick surface that repels water also repels adhesives. Bonding PTFE to other materials is notoriously difficult.
Achieving a strong bond requires specialized surface preparation, such as chemical etching, which alters the surface chemistry to create anchor points for an adhesive. This adds complexity and cost to manufacturing processes.
Mechanical Limitations
While chemically robust, PTFE is a relatively soft material. It is susceptible to creep (or "cold flow"), where the material slowly deforms under sustained pressure.
In applications involving high mechanical loads or abrasive conditions, its integrity as a water barrier could be compromised over time if the design does not account for these mechanical weaknesses.
Making the Right Choice for Your Application
Selecting a material requires balancing its benefits against its limitations in the context of your specific goal.
- If your primary focus is a waterproof and chemically inert barrier: PTFE is an elite choice, especially as a coating, liner, or seal where mechanical stress is managed.
- If your primary focus is a structural part in a wet environment: Carefully evaluate the mechanical loads, as pure PTFE may deform. Filled grades of PTFE can improve strength and creep resistance.
- If your primary focus requires adhering a water-resistant surface to another material: Factor in the necessity and cost of specialized surface treatments like chemical etching to achieve a reliable bond.
Ultimately, understanding the molecular origins of PTFE's water resistance empowers you to leverage its remarkable barrier properties while respecting its inherent mechanical and adhesive limitations.
Summary Table:
| Property | Description | Benefit |
|---|---|---|
| Molecular Structure | Carbon backbone shielded by a dense sheath of fluorine atoms. | Creates a chemically inert, non-polar surface. |
| Surface Energy | Extremely low surface energy. | Strongly repels polar liquids like water, causing beading. |
| Water Absorption | Negligible (typically < 0.01%). | Maintains dimensional stability and electrical properties in wet environments. |
| Chemical Resistance | Excellent barrier against corrosive fluids. | Ideal for liners and seals in harsh chemical and water applications. |
Leverage PTFE's Superior Water Resistance for Your Application
At KINTEK, we specialize in manufacturing high-precision PTFE components—including seals, liners, and custom labware—that harness these exact molecular properties to deliver unmatched performance in wet and corrosive environments. Whether you're in the semiconductor, medical, laboratory, or industrial sector, our expertise in custom fabrication from prototypes to high-volume orders ensures you get a solution that provides a reliable, long-lasting barrier.
Ready to solve your sealing or contamination challenges? Contact our experts today to discuss how our precision PTFE components can enhance your product's reliability and longevity.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Teflon Balls for Advanced Industrial Applications
People Also Ask
- How does PTFE contribute to low friction and wear resistance? Achieve Superior Performance with Advanced Materials
- Why is PTFE rod suitable for automotive applications? Boost Vehicle Performance & Durability
- Why is CNC machining preferred for Teflon parts over other methods? Unlock Precision & Complex Designs
- What are the future considerations for machining Teflon? Mastering Material Challenges with Smart Tech
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications



















