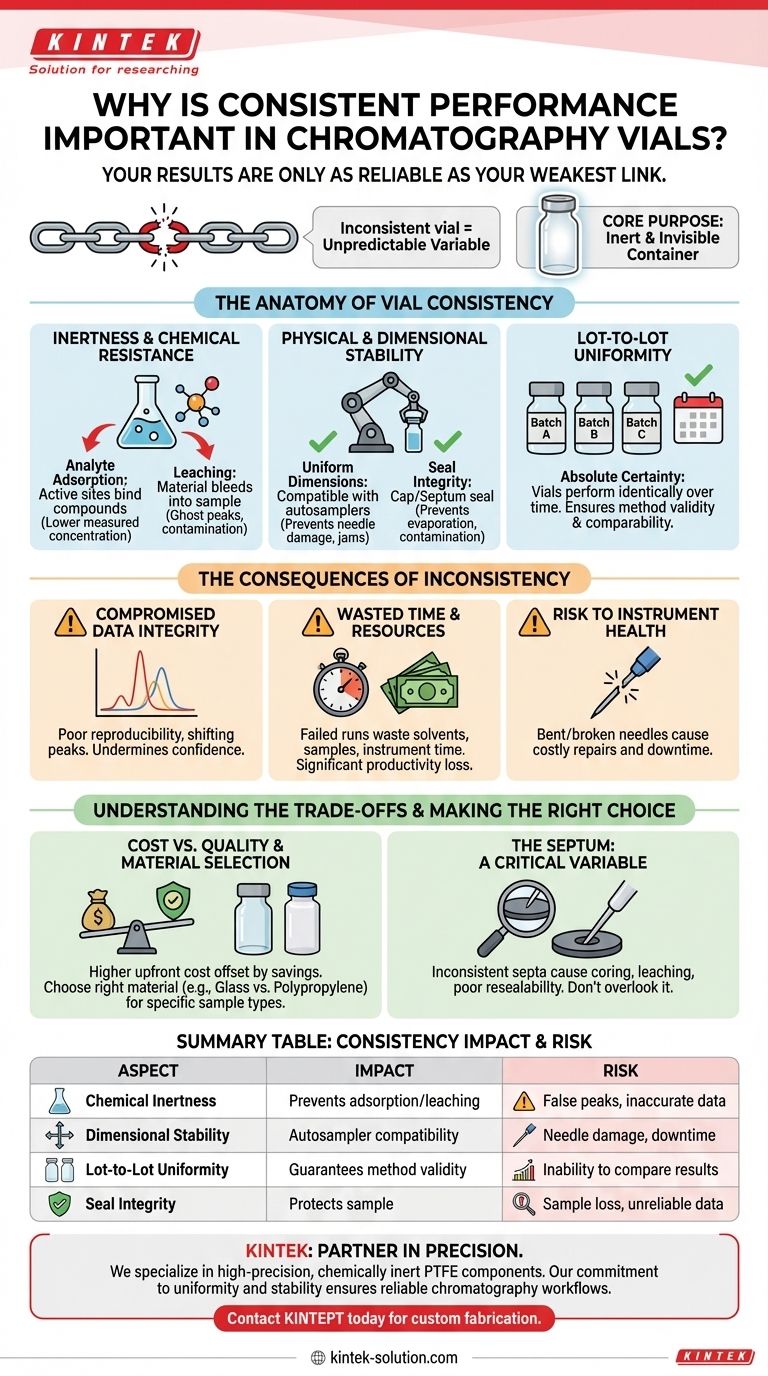
In analytical science, your results are only as reliable as your weakest link. Consistent performance in chromatography vials is essential because it ensures the vial itself does not become an unpredictable variable in your analysis, directly safeguarding the accuracy and reproducibility of your data. Without it, you cannot trust that changes in results are due to your sample, not your container.
The core purpose of a chromatography vial is to be a completely inert and invisible container for your sample. Consistency means that every vial, from the first to the last and from batch to batch, performs this role perfectly, preventing it from introducing errors that compromise your entire analytical workflow.

The Anatomy of Vial Consistency
True consistency is not a single feature but a combination of several critical material and manufacturing attributes. Each one plays a role in protecting the integrity of your sample from the moment it's prepared to the moment it's injected.
Inertness and Chemical Resistance
A vial must not interact with your sample. This is the most fundamental requirement.
Inconsistent chemical resistance can lead to analyte adsorption, where active sites on the glass or plastic surface bind to your compounds of interest, artificially lowering their measured concentration.
Conversely, leaching can occur, where compounds from the vial material or manufacturing process bleed into your sample, creating ghost peaks and contaminating your analysis.
Physical and Dimensional Stability
The physical specifications of a vial are critical for automated systems.
Vials must have uniform dimensions—height, diameter, and neck finish—to be compatible with autosampler trays and robotic arms. An out-of-spec vial can cause needle damage, missed injections, or system jams.
The integrity of the cap and septum seal is also a physical property. A consistent seal prevents sample evaporation and protects against atmospheric contamination.
Lot-to-Lot Uniformity
Perhaps the most crucial factor for long-term studies is uniformity between manufacturing batches.
You need absolute certainty that the box of vials you use next month will perform identically to the one you are using today. This ensures that your method remains valid and that results from different time points can be reliably compared.
The Consequences of Inconsistency
Using inconsistent or low-quality vials introduces significant risks that extend beyond a single bad result. These consequences can impact project timelines, budgets, and even the laboratory's reputation.
Compromised Data Integrity
This is the most severe consequence. Inconsistent vials lead to poor reproducibility, shifting peak areas, and unexplained artifacts. This forces analysts to question every result, undermining confidence in the data.
Wasted Time and Resources
A single failed analytical run due to a vial issue wastes expensive solvents, consumes valuable and often irreplaceable samples, and ties up instrument time.
The subsequent troubleshooting effort to identify the source of the problem—was it the sample, the method, or the vial?—represents a significant loss of productivity.
Risk to Instrument Health
Inconsistent physical dimensions can cause direct harm to your equipment.
Bent or broken autosampler needles are a common result of out-of-spec vials. These incidents not only require costly repairs but also lead to significant instrument downtime, halting all work.
Understanding the Trade-offs
Choosing the right vial involves balancing performance requirements with practical constraints. Acknowledging these trade-offs is key to making an informed decision.
Cost vs. Quality
Certified, high-performance vials often have a higher upfront cost. However, this initial investment is frequently offset by the significant savings from avoiding failed runs, instrument downtime, and time-consuming troubleshooting.
Viewing vials as a consumable can be a false economy. It is more accurate to see them as a critical component of data quality assurance.
Material Selection Matters
Not all "consistent" vials are suitable for every application. Type 1 borosilicate glass is a standard for its inertness, but it can be problematic for high-pH samples.
Similarly, polypropylene vials offer excellent resistance to certain chemicals but may not have the same thermal stability as glass. Consistency within the wrong material type will still lead to poor results.
The Septum: An Overlooked Variable
The vial is a system, and the septum is a critical part of it. An inconsistent septum can introduce just as many problems as the vial itself.
Issues like coring (where needle pierces a piece of the septum into the sample), leaching of plasticizers, or poor resealability after puncture can all compromise sample integrity and introduce variability.
Making the Right Choice for Your Analysis
To ensure reliable performance, select vials based on the specific demands of your analytical method and goals.
- If your primary focus is trace analysis or LC-MS: Prioritize certified low-adsorption and low-bleed vials to minimize background noise and maximize analyte recovery.
- If your primary focus is high-throughput screening: Emphasize certified dimensional consistency to prevent autosampler failures and ensure uninterrupted, reliable operation.
- If your primary focus is working with aggressive solvents or pH extremes: Verify the chemical resistance of both the vial material (e.g., borosilicate glass vs. polypropylene) and the septa.
By treating the chromatography vial as a critical component, you safeguard the integrity of your entire analytical workflow.
Summary Table:
| Aspect of Consistency | Impact on Analysis | Risk of Inconsistency |
|---|---|---|
| Chemical Inertness | Prevents analyte adsorption and leaching | False peaks, inaccurate concentrations, data corruption |
| Dimensional Stability | Ensures compatibility with autosamplers | Needle damage, missed injections, instrument downtime |
| Lot-to-Lot Uniformity | Guarantees method validity over time | Inability to compare results from different batches or studies |
| Seal Integrity | Protects against evaporation and contamination | Sample loss, atmospheric interference, unreliable data |
Stop risking your data integrity with inconsistent vials. KINTEK specializes in manufacturing high-precision, chemically inert PTFE components, including custom vial inserts and seals, for the most demanding laboratory and semiconductor applications. Our commitment to lot-to-lot uniformity and dimensional stability ensures your chromatography workflows are reliable, reproducible, and free from vial-related variables.
Let us be your partner in precision. Contact KINTEPT today to discuss how our custom fabrication services—from prototypes to high-volume orders—can safeguard your analytical results.
Visual Guide
Related Products
- PTFE Chemical Solvent Sampling Spoon
- Customizable PTFE Crucibles for Laboratory and Industrial Applications
- Customizable PTFE Three Neck Flasks for Advanced Chemical Applications
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Bottles for Diverse Industrial Applications
People Also Ask
- What industries commonly use PTFE and why? Unlock the Power of PTFE for Extreme Environments
- What are the features of PTFE laboratory bottles? Unmatched Chemical Resistance & Extreme Temperature Tolerance
- What is PTFE and what are its basic properties? The Ultimate Guide to the High-Performance Polymer
- What are the unique properties of PTFE that make it commercially valuable? Unlock Unmatched Performance
- What temperature range can PTFE vials withstand? From -200°C to +260°C for Extreme Applications



















