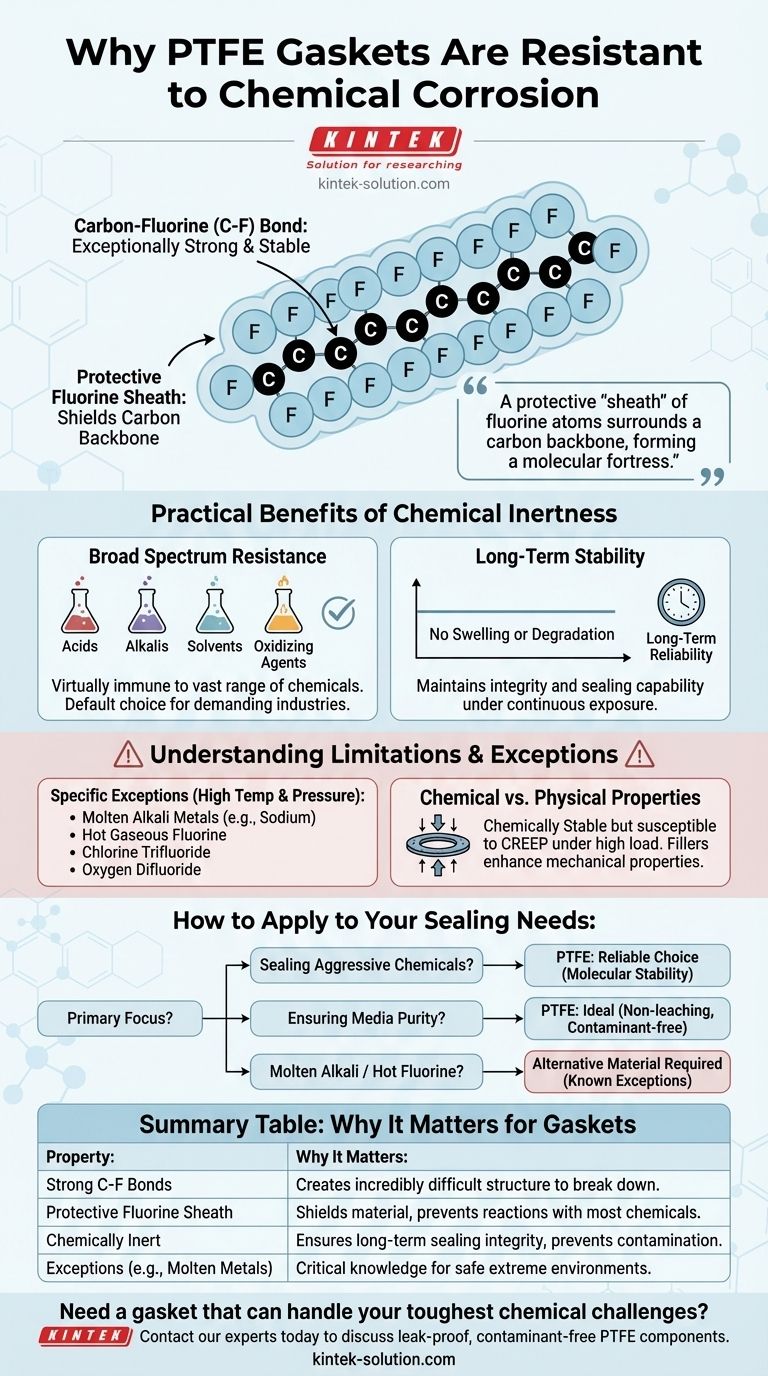
The exceptional chemical resistance of PTFE gaskets stems directly from their unique molecular structure. This structure is defined by extremely strong, stable bonds between carbon and fluorine atoms, which create a chemically inert material that does not react with the vast majority of substances.
The core reason for PTFE's resilience is not a coating or treatment, but its fundamental chemistry. A protective "sheath" of fluorine atoms surrounds a carbon backbone, forming a molecular fortress that is incredibly difficult for other chemicals to penetrate or break down.

The Molecular Foundation of PTFE's Resilience
To understand why PTFE (Polytetrafluoroethylene) is so non-reactive, we must look at its atomic composition. The material is a polymer made of a long chain of carbon atoms, where each carbon is bonded to two fluorine atoms.
The Carbon-Fluorine Bond: A Chemical Fortress
The bond between carbon and fluorine (C-F) is one of the strongest single bonds in organic chemistry.
Fluorine is the most electronegative element, meaning it holds onto its electrons very tightly. This creates a very short, strong, and stable bond with carbon that requires a massive amount of energy to break.
The Protective Fluorine Sheath
The fluorine atoms are larger than the carbon atoms they are bonded to. This causes them to arrange themselves in a tight, helical sheath around the carbon backbone.
This dense outer layer of non-reactive fluorine atoms effectively shields the vulnerable carbon chain from outside chemical attack. Most chemicals simply cannot get close enough to initiate a reaction.
What "Chemically Inert" Means in Practice
This molecular stability translates directly into superior performance in demanding industrial applications where chemical exposure is constant.
Broad Spectrum Resistance
PTFE gaskets are virtually immune to a vast range of chemicals. This includes highly aggressive acids, alkalis, solvents, and oxidizing agents.
This property makes PTFE a default choice for sealing applications in chemical processing, pharmaceuticals, and any industry handling corrosive or hazardous materials.
Long-Term Stability
Unlike other materials that may swell, soften, or degrade over time, PTFE remains stable.
It does not lose its integrity or sealing capability, even after continuous exposure to harsh chemicals, ensuring long-term reliability and safety.
Understanding the Limitations and Exceptions
While its resistance is remarkable, no material is universally perfect. Understanding the few specific exceptions to PTFE's chemical inertness is critical for safe and effective application.
The Few Chemicals That Can Attack PTFE
Only a very small and specific group of substances can corrode PTFE, and typically only under specific conditions like high temperatures.
These exceptions include molten alkali metals (like sodium), hot gaseous fluorine, and potent fluorinating agents such as chlorine trifluoride and oxygen difluoride. For nearly all other applications, PTFE remains completely inert.
The Difference Between Chemical and Physical Properties
It is important to distinguish chemical resistance from physical properties. While chemically stable, pure PTFE can be susceptible to creep or "cold flow" under high pressure and temperature.
This is a physical deformation, not a chemical breakdown. Fillers are often added to PTFE (creating "filled PTFE") to enhance its mechanical properties and resistance to cold flow while retaining its chemical inertness.
How to Apply This to Your Sealing Needs
Your choice of gasket material should be based on a clear understanding of the chemical environment it will face.
- If your primary focus is sealing aggressive acids, bases, or solvents: PTFE is an exceptionally safe and reliable choice due to its fundamental molecular stability.
- If your primary focus is ensuring media purity: PTFE's non-reactive nature prevents it from leaching or contaminating the process fluid, making it ideal for food, pharma, and semiconductor applications.
- If your application involves molten alkali metals or specific hot fluorine compounds: You must select an alternative material, as these are the known, rare exceptions to PTFE's chemical resistance.
Ultimately, understanding the "why" behind a material's properties empowers you to select the most reliable and safest solution for your specific challenge.
Summary Table:
| Property | Why It Matters for Gaskets |
|---|---|
| Strong C-F Bonds | Creates a molecular structure that is incredibly difficult for chemicals to break down. |
| Protective Fluorine Sheath | Shields the material, preventing reactions with most acids, bases, and solvents. |
| Chemically Inert | Ensures long-term sealing integrity and prevents contamination of process media. |
| Exceptions (e.g., Molten Alkali Metals) | Critical to know for safe application in extreme environments. |
Need a gasket that can handle your toughest chemical challenges?
At KINTEK, we leverage PTFE's inherent chemical resistance to manufacture high-performance seals, liners, and labware for the semiconductor, medical, laboratory, and industrial sectors. Whether you require a standard component or a custom-fabricated solution from prototype to high-volume production, our precision manufacturing ensures reliability and purity.
Contact our experts today to discuss how our PTFE components can provide a leak-proof, contaminant-free seal for your critical applications.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
People Also Ask
- What industries use PTFE machined parts and for what applications? Critical Components for Demanding Environments
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications
- Why is CNC machining preferred for Teflon parts over other methods? Unlock Precision & Complex Designs
- Why is PTFE rod suitable for automotive applications? Boost Vehicle Performance & Durability
- What are the future considerations for machining Teflon? Mastering Material Challenges with Smart Tech



















