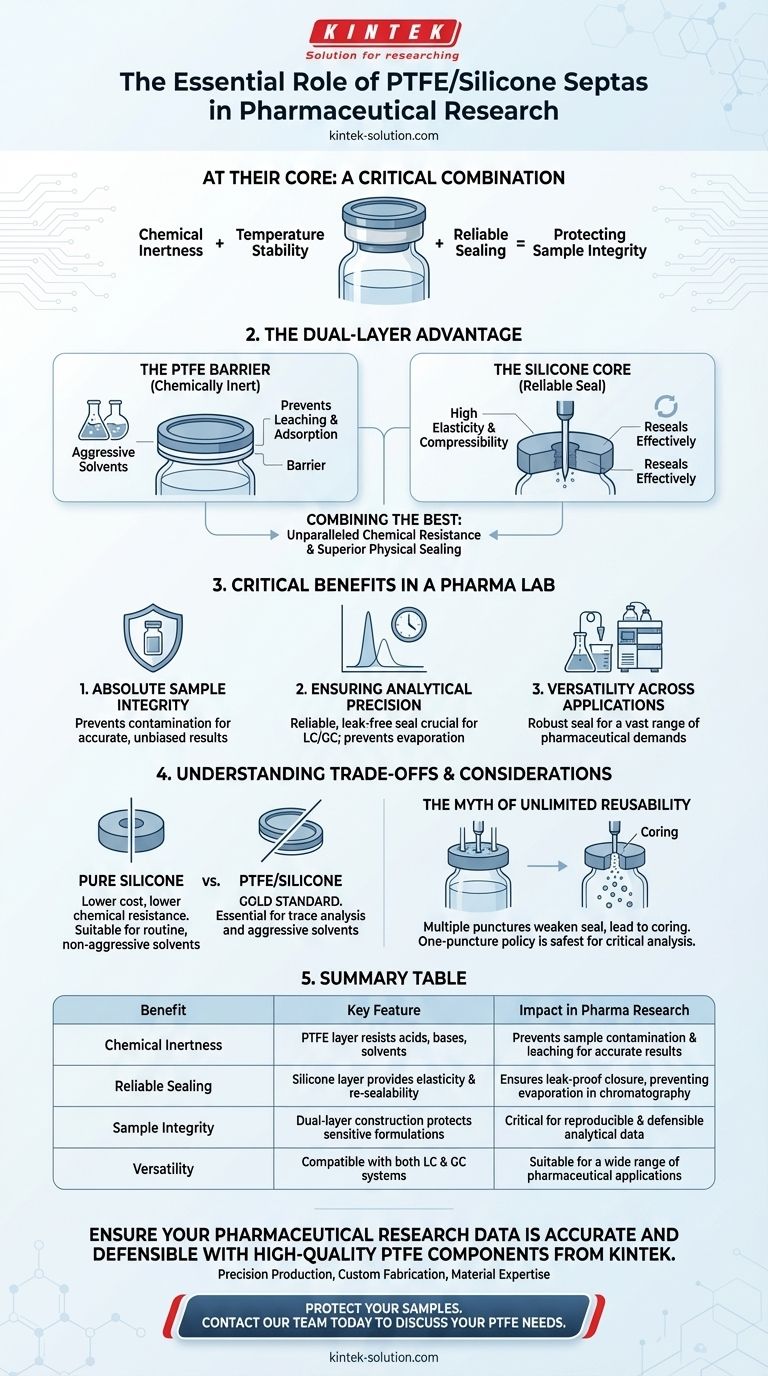
At their core, PTFE/silicone septa provide a critical combination of chemical inertness, temperature stability, and reliable sealing, making them essential for protecting sample integrity in pharmaceutical research. Their unique dual-layer construction ensures that sensitive drug formulations are not contaminated during storage or analysis, which is fundamental for generating accurate and reproducible results in applications like chromatography.
The crucial insight is understanding that PTFE/silicone septa are not a single material, but a composite system. The PTFE layer provides a chemically non-reactive barrier against your sample, while the silicone layer provides the mechanical elasticity for a perfect, re-sealable seal.

The Dual-Layer Advantage: How PTFE and Silicone Work Together
To understand the value of these septa, you must first understand their construction. They are not simply a blend of materials but a strategic combination of two distinct layers, each serving a critical purpose.
The PTFE Barrier: Ensuring Chemical Inertness
The layer that faces your sample is a thin film of polytetrafluoroethylene (PTFE). This material is exceptionally non-reactive.
It is compatible with the acidic, alkaline, and aggressive organic solvents commonly used in pharmaceutical analysis. This inertness is non-negotiable, as it prevents chemicals from leaching from the septa into your sample or analytes from adsorbing onto the septa surface.
The Silicone Core: Providing a Reliable Seal
Behind the PTFE film is a thicker layer of silicone. Silicone's primary benefit is its high elasticity and compressibility.
This property allows the septa to form a tight, leak-proof seal against the rim of a vial. When punctured by an autosampler needle, the silicone's elasticity also allows it to reseal effectively, preventing sample evaporation and atmospheric contamination.
Combining the Best of Both Worlds
This dual-layer design delivers a solution that is superior to either material alone. You get the unparalleled chemical resistance of PTFE with the superior physical sealing and resealing capabilities of silicone. This ensures the integrity of the sample from storage through injection.
Critical Benefits in a Pharmaceutical Lab
The properties of PTFE/silicone septa directly translate into tangible benefits that address the stringent demands of pharmaceutical quality control and research.
Maintaining Absolute Sample Integrity
The primary goal is to ensure the sample inside the vial is the only thing being analyzed. PTFE/silicone septa prevent contamination from the closure, ensuring that the analytical results for a drug formulation are accurate and unbiased.
Ensuring Analytical Precision
In quantitative techniques like liquid and gas chromatography (LC/GC), even minor sample evaporation can alter the concentration and lead to inaccurate results. The reliable, leak-free seal provided by these septa is crucial for maintaining the precise concentration of the sample over time.
Versatility Across Applications
The combination of chemical inertness and a robust seal makes these septa the default choice for a vast range of pharmaceutical applications. They perform reliably in both GC and LC systems, which have different operational demands.
Understanding the Trade-offs and Considerations
While PTFE/silicone is the gold standard for many applications, it's important to understand its context and limitations to make informed decisions.
Pure Silicone vs. PTFE/Silicone
Pure silicone septa exist as a lower-cost alternative. However, silicone has significantly lower chemical resistance than PTFE. It is only suitable for routine procedures involving non-aggressive solvents, such as water or methanol, where the risk of chemical interaction is minimal.
The Myth of Unlimited Reusability
While the ability to reseal is a key feature, it is not infinite. Each puncture creates a physical hole that weakens the seal. Re-puncturing the septa multiple times, especially in the same spot, can lead to coring—where tiny particles of the septa break off and fall into the sample—or a compromised seal. For critical analyses, a one-puncture policy is the safest approach.
The Importance of a Proper Fit
The effectiveness of any septa is dependent on its fit within the cap and its compression against the vial. Using the wrong size or improperly torquing the cap can negate the benefits of a high-quality septum, leading to leaks and sample evaporation.
Making the Right Choice for Your Analysis
Your choice of septa should be directly guided by the sensitivity of your analysis and the nature of your sample.
- If your primary focus is trace analysis or aggressive solvents: PTFE/silicone is the only acceptable choice to prevent sample contamination and ensure data integrity.
- If your primary focus is high-throughput screening with automated systems: Use pre-slit PTFE/silicone septa to reduce needle strain, minimize coring, and ensure consistent penetration.
- If your primary focus is routine analysis with mild, aqueous solvents: Pure silicone may be a cost-effective option, but it must be validated to ensure no analyte loss or contamination occurs.
Ultimately, selecting the correct septa is a foundational step in generating reliable and defensible analytical data.
Summary Table:
| Benefit | Key Feature | Impact in Pharma Research |
|---|---|---|
| Chemical Inertness | PTFE layer resists acids, bases, and solvents | Prevents sample contamination and leaching for accurate results |
| Reliable Sealing | Silicone layer provides elasticity and re-sealability | Ensures leak-proof closure, preventing evaporation in chromatography |
| Sample Integrity | Dual-layer construction protects sensitive formulations | Critical for reproducible and defensible analytical data |
| Versatility | Compatible with both LC and GC systems | Suitable for a wide range of pharmaceutical applications |
Ensure your pharmaceutical research data is accurate and defensible with high-quality PTFE components from KINTEK.
As specialists in precision PTFE manufacturing for the semiconductor, medical, and laboratory industries, we understand the critical need for chemical inertness and reliable performance in your analytical processes. Whether you require standard PTFE/silicone septa or custom-fabricated components for unique applications, KINTEK delivers:
- Precision Production: Consistent, high-quality PTFE parts that meet stringent industry standards.
- Custom Fabrication: Tailored solutions from prototypes to high-volume orders to fit your specific vial and cap configurations.
- Material Expertise: Deep knowledge of PTFE and composite materials for demanding pharmaceutical environments.
Protect your samples and your data. Contact our team today to discuss your PTFE component needs and request a quote.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- PTFE Chemical Solvent Sampling Spoon
- Custom PTFE Bottles for Diverse Industrial Applications
People Also Ask
- Why are PTFE vials considered environmentally friendly? Reduce Lab Waste with Durable Reusables
- What are the key properties of PTFE for sanitary gaskets? Ensuring Purity & Safety in Food & Pharma
- How does Teflon contribute to the reliability of medical equipment? Ensuring Longevity and Safety
- What are the advantages of PTFE lids for jacketed and process vessels? Achieve Superior Durability & Chemical Resistance
- What are the applications of PTFE-lined bottle caps? Ensure Ultimate Purity and Chemical Resistance
- How does applying a fluoropolymer film improve pharmaceutical stoppers? Enhance Drug Safety and Stability
- What are the main advantages of PTFE as a material for laboratory bottles? Superior Chemical & Thermal Resistance
- What are the benefits of selecting the appropriate PTFE-coated septum for chromatography? Ensure Accurate & Reproducible Results



















