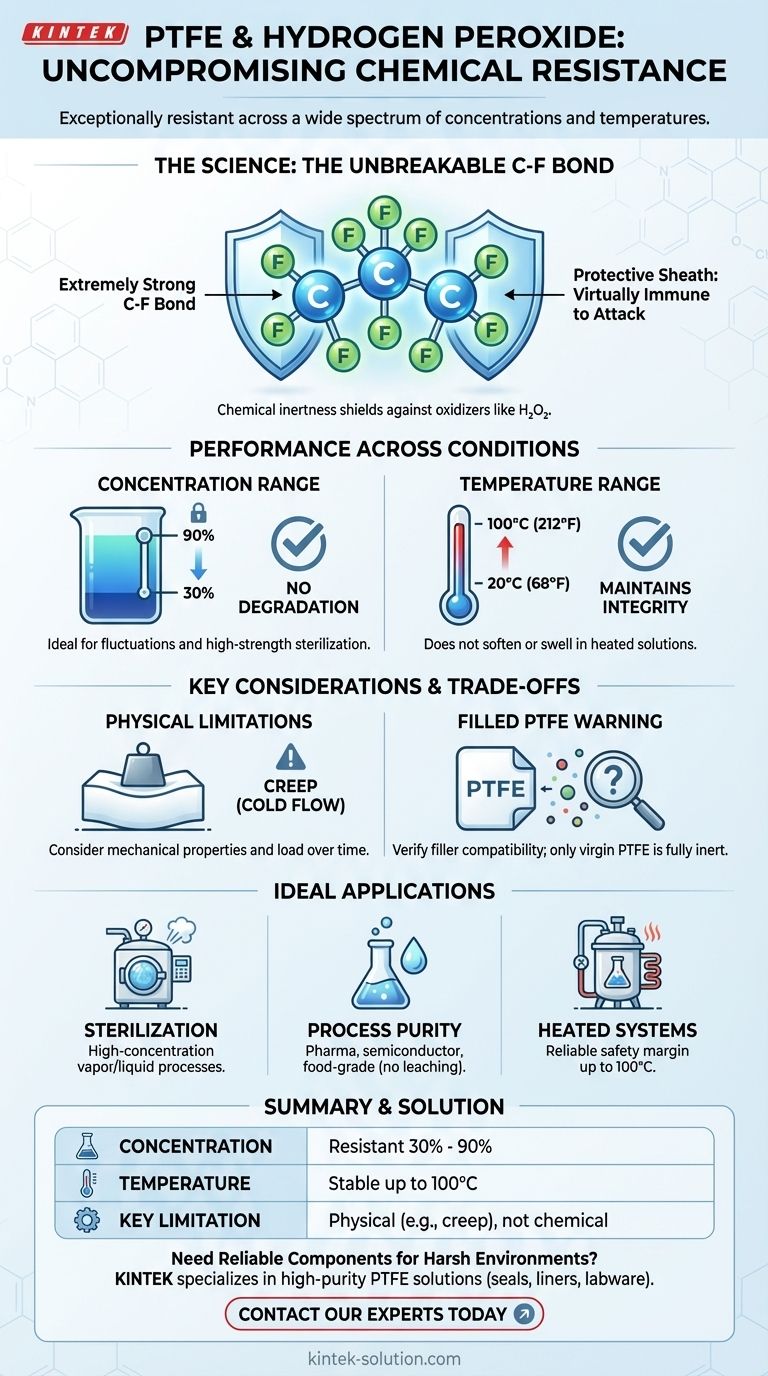
In short, Polytetrafluoroethylene (PTFE) is exceptionally resistant to hydrogen peroxide. This resistance holds true across a wide spectrum of concentrations, from 30% all the way up to 90%, and is maintained at temperatures as high as 100°C (212°F). The material's fundamental chemistry makes it one of the most reliable choices for applications involving this powerful oxidizing agent.
The core reason for PTFE's performance is its chemical inertness, which is a direct result of its extremely strong carbon-fluorine bonds. This molecular structure makes PTFE virtually immune to chemical attack from hydrogen peroxide, regardless of concentration or temperature within its operational limits.
The Foundation of PTFE's Chemical Resistance
The remarkable compatibility of PTFE with aggressive chemicals like hydrogen peroxide isn't an accident; it's a feature of its molecular architecture.
The Unbreakable Carbon-Fluorine Bond
At the heart of PTFE's stability is the carbon-fluorine (C-F) bond, which is one of the strongest single bonds in organic chemistry.
The fluorine atoms create a tight, protective "sheath" around the carbon backbone of the polymer. This sheath effectively shields the carbon chain from attack by outside chemical agents, including potent oxidizers like hydrogen peroxide.
A Non-Reactive Surface
This molecular structure renders PTFE almost universally inert. It resists nearly all acids, bases, and solvents.
For a chemical reaction to occur, the hydrogen peroxide would need to break the C-F bonds, an act that requires a significant amount of energy. Under typical industrial conditions, this simply does not happen.
Performance Across a Range of Conditions
Data confirms that PTFE's resistance is not limited to specific, narrow conditions. It performs reliably across a broad operational window.
Impact of Concentration
PTFE shows no degradation when exposed to hydrogen peroxide at both 30% and very high (30-90%) concentrations.
This makes it an ideal material for systems that may see fluctuations in concentration or that are used for sterilization processes involving high-strength hydrogen peroxide vapor or liquid.
Impact of Temperature
This chemical resistance is maintained across a practical temperature range, with documented performance at 20°C (68°F), 60°C (140°F), and 100°C (212°F).
The material does not soften, swell, or lose its integrity when exposed to heated hydrogen peroxide solutions, a critical factor for many industrial processes.
Key Considerations and Trade-offs
While chemically robust, no material is without its limitations. For PTFE, these are almost always physical, not chemical.
Physical Properties Matter
PTFE is a relatively soft material. Its primary limitations are mechanical. You must consider factors like creep (cold flow), where the material can deform over time under a constant load, especially at elevated temperatures.
While chemically impervious, its physical structure can be a factor in high-pressure sealing applications.
Purity and Additives
The exceptional resistance described applies to pure, virgin PTFE. Many commercial PTFE components are filled with other materials (like glass, carbon, or bronze) to improve their mechanical properties.
If you are using a filled PTFE, you must verify the chemical compatibility of the filler material itself with hydrogen peroxide, as the filler may not share PTFE's inertness.
Making the Right Choice for Your Application
Selecting a material requires matching its properties to your primary goal. PTFE is an excellent choice when chemical stability is paramount.
- If your primary focus is high-concentration sterilization: PTFE is an industry-standard choice due to its proven resistance to the high-strength hydrogen peroxide used in these processes.
- If your primary focus is process purity: The inertness of virgin PTFE ensures it will not leach contaminants into your system, making it ideal for pharmaceutical, semiconductor, or food-grade applications.
- If your primary focus is performance in heated systems: PTFE's ability to withstand hydrogen peroxide at temperatures up to 100°C (212°F) provides a reliable safety margin for heated chemical processes.
For applications demanding uncompromising chemical resistance to hydrogen peroxide, PTFE remains a first-tier engineering material.

Summary Table:
| Condition | PTFE Performance |
|---|---|
| Concentration | Resistant from 30% to 90% |
| Temperature | Stable up to 100°C (212°F) |
| Key Limitation | Physical properties (e.g., creep), not chemical resistance |
Need a reliable component for harsh chemical environments? KINTEK specializes in manufacturing high-purity PTFE components (seals, liners, labware, etc.) that offer exceptional resistance to hydrogen peroxide and other aggressive chemicals. Our expertise in custom fabrication—from prototypes to high-volume orders—ensures you get the perfect solution for your semiconductor, medical, laboratory, or industrial application. Contact our experts today to discuss your specific requirements!
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- PTFE Deep Evaporating Dishes Customizable Laboratory and Industrial Solutions
- PTFE Chemical Solvent Sampling Spoon
People Also Ask
- What are the main advantages of using PTFE parts in industrial applications? Unlock Unmatched Chemical Resistance and Reliability
- What tips can improve Teflon machining results? Master Sharp Tools, Heat Control, and Rigid Support
- What industries use PTFE machined parts and for what applications? Critical Components for Demanding Environments
- Why is CNC machining preferred for Teflon parts over other methods? Unlock Precision & Complex Designs
- Why is PTFE rod suitable for automotive applications? Boost Vehicle Performance & Durability



















