At its core, the remarkable chemical resistance of Polytetrafluoroethylene (PTFE) is a direct result of its molecular structure. This is due to the incredibly strong and stable bonds between carbon and fluorine atoms. These bonds form a protective, non-reactive "sheath" around the polymer's carbon backbone, effectively shielding it from attack by almost all chemicals, acids, and solvents.
The chemical inertness of PTFE is not a complex phenomenon but a simple matter of atomic bonding. The strength and stability of the carbon-fluorine bond are so immense that very few substances have the energy to break it, making the material virtually impenetrable to chemical attack.

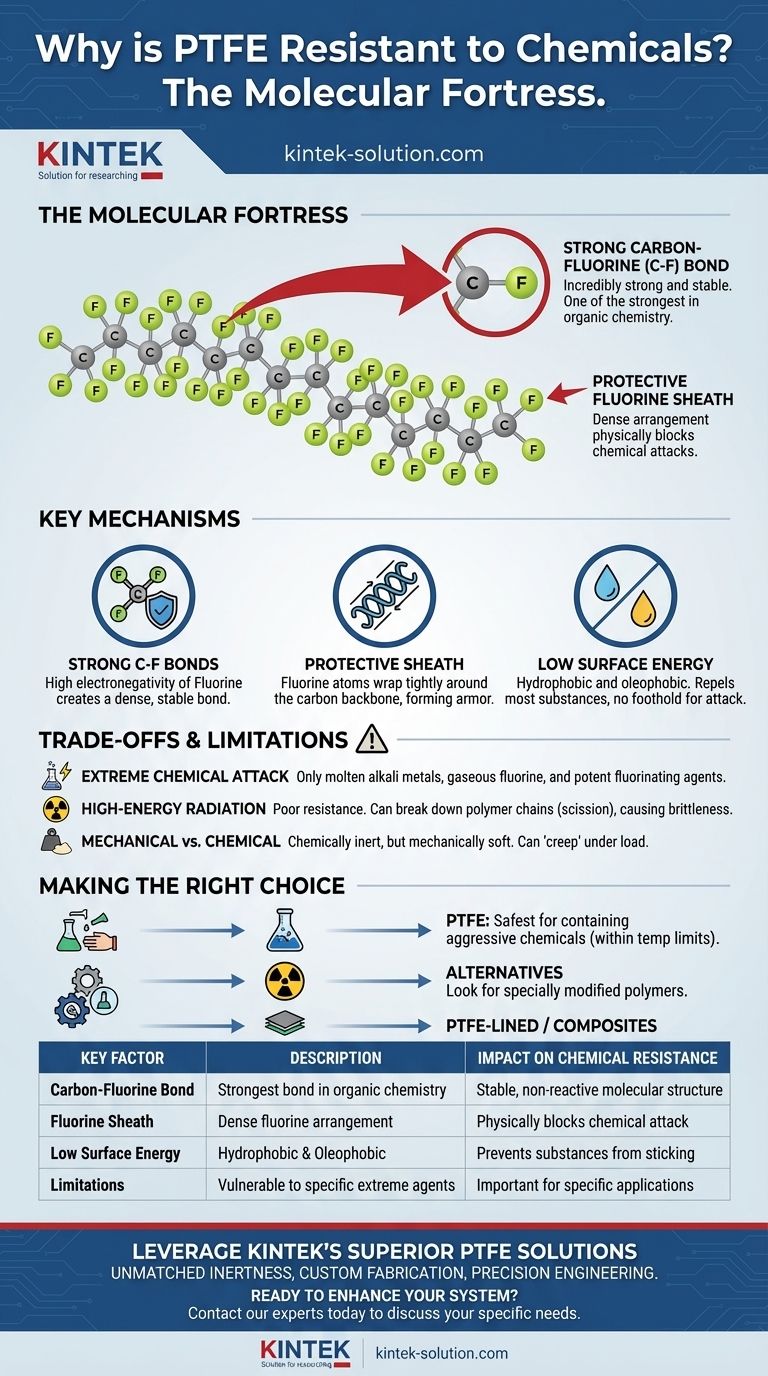
The Molecular Fortress: Why PTFE Resists Attack
To truly grasp PTFE's resilience, we must look at its atomic composition. The properties that make it so valuable are not accidental; they are a direct consequence of fundamental chemistry.
The Carbon-Fluorine Bond: The Key to Inertness
PTFE is a polymer, which means it consists of a long chain of repeating units. The backbone of this chain is made of carbon atoms.
What makes PTFE unique is that each carbon atom is bonded to two fluorine atoms. The carbon-fluorine (C-F) bond is one of the strongest single bonds in organic chemistry.
Fluorine is the most electronegative element, meaning it has an extremely strong pull on electrons. This creates a very short, dense, and stable bond with carbon that is difficult to break.
The Protective Fluorine Sheath
The fluorine atoms are also relatively large compared to the carbon atoms they are bonded to. They wrap tightly around the carbon backbone in a helical formation.
This dense arrangement of fluorine atoms creates a protective "sheath" or "armor" around the vulnerable carbon chain. This sheath physically blocks almost all chemicals from getting close enough to react with the polymer's backbone.
Low Surface Energy
Because the fluorine atoms hold their electrons so tightly, the surface of PTFE has very little free energy. It is electrically neutral and non-polar.
This is why other substances do not "stick" to PTFE. The material is both hydrophobic (repels water) and oleophobic (repels oil), leaving no way for most chemical agents to gain a foothold for an attack.
Understanding the Trade-offs and Limitations
While PTFE's chemical resistance is legendary, no material is perfect. Acknowledging its limits is crucial for proper application.
When PTFE Can Be Attacked
Only a handful of extremely aggressive substances can compromise PTFE. These include molten alkali metals (like sodium), gaseous fluorine, and potent fluorinating agents like chlorine trifluoride.
These materials are uniquely reactive and possess enough energy to disrupt the powerful carbon-fluorine bond. For the vast majority of industrial and laboratory applications, these exceptions are not a concern.
Poor Resistance to High-Energy Radiation
PTFE has relatively poor resistance to high-energy radiation, such as gamma rays or electron beams. This type of radiation doesn't attack the material chemically but can break down the polymer chains themselves.
This process, known as scission, causes the material to become brittle and lose its mechanical integrity, even while it remains chemically inert.
Mechanical vs. Chemical Properties
It is vital to distinguish between chemical resistance and mechanical strength. PTFE is a relatively soft material with a tendency to "creep" or deform under sustained load.
While it won't be degraded by a corrosive chemical, its suitability for a high-pressure or high-abrasion environment depends on the mechanical, not just the chemical, demands of the application.
Making the Right Choice for Your Goal
Understanding these principles allows you to specify materials with confidence. Your decision should be guided by the primary challenge you need to solve.
- If your primary focus is containing aggressive chemicals: PTFE is almost always the safest and most reliable choice, as long as you operate within its temperature limits and avoid its few known chemical vulnerabilities.
- If your application involves high radiation: You must look for alternatives or specially modified grades of polymers, as standard PTFE will degrade and lose its structural integrity.
- If you need both chemical resistance and high mechanical strength: Consider PTFE-lined metal components or composite materials, which combine PTFE's inertness with the rigidity of other materials.
By understanding the molecular basis of PTFE's resilience, you can leverage its power with precision and confidence.
Summary Table:
| Key Factor | Description | Impact on Chemical Resistance |
|---|---|---|
| Carbon-Fluorine Bond | One of the strongest bonds in organic chemistry | Provides a stable, non-reactive molecular structure |
| Fluorine Sheath | Dense arrangement of fluorine atoms around carbon backbone | Physically blocks chemicals from attacking the polymer |
| Low Surface Energy | Hydrophobic and oleophobic properties | Prevents substances from sticking or gaining a foothold |
| Limitations | Vulnerable to molten alkali metals, gaseous fluorine, and high-energy radiation | Important to consider for specific applications |
Leverage PTFE's Superior Chemical Resistance for Your Critical Applications
At KINTEK, we specialize in manufacturing high-precision PTFE components—including seals, liners, and custom labware—for industries where chemical inertness is non-negotiable. Whether you're in semiconductor manufacturing, medical device production, or laboratory research, our expertise ensures your components withstand the most aggressive environments.
Our PTFE solutions offer:
- Unmatched Chemical Inertness: Ideal for handling acids, solvents, and corrosive substances.
- Custom Fabrication: From prototypes to high-volume orders, tailored to your exact specifications.
- Precision Engineering: Components designed for reliability and performance under demanding conditions.
Ready to enhance your system's durability and safety? Contact our experts today to discuss your specific needs and discover how KINTEK's PTFE components can protect your processes and products.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
People Also Ask
- What are the basic materials used in slide bearings? Explore PTFE, Composites & Multi-Layer Designs
- How do PTFE slide bearings compare in load capacity to traditional bearings? Superior Support for High-Load, Low-Speed Applications
- Which regulatory standards do PTFE rotary shaft seals comply with? Ensure Compliance for Your Application
- What are the benefits of PTFE in electrical generation and distribution? Ensure Safety and Reliability
- Which industries commonly use PTFE cryogenic seals? Essential for Aerospace, Oil & Gas, and Pharma
- What are the limitations of virgin PTFE in high-temperature applications? Avoid Creep and Sealing Failures
- What are the structural differences between PTFE oil seals and rubber oil seals? A Guide to Lip Design & Performance
- What are the advantages of PTFE laminates in RF applications? Achieve Superior Signal Integrity and Reliability



















