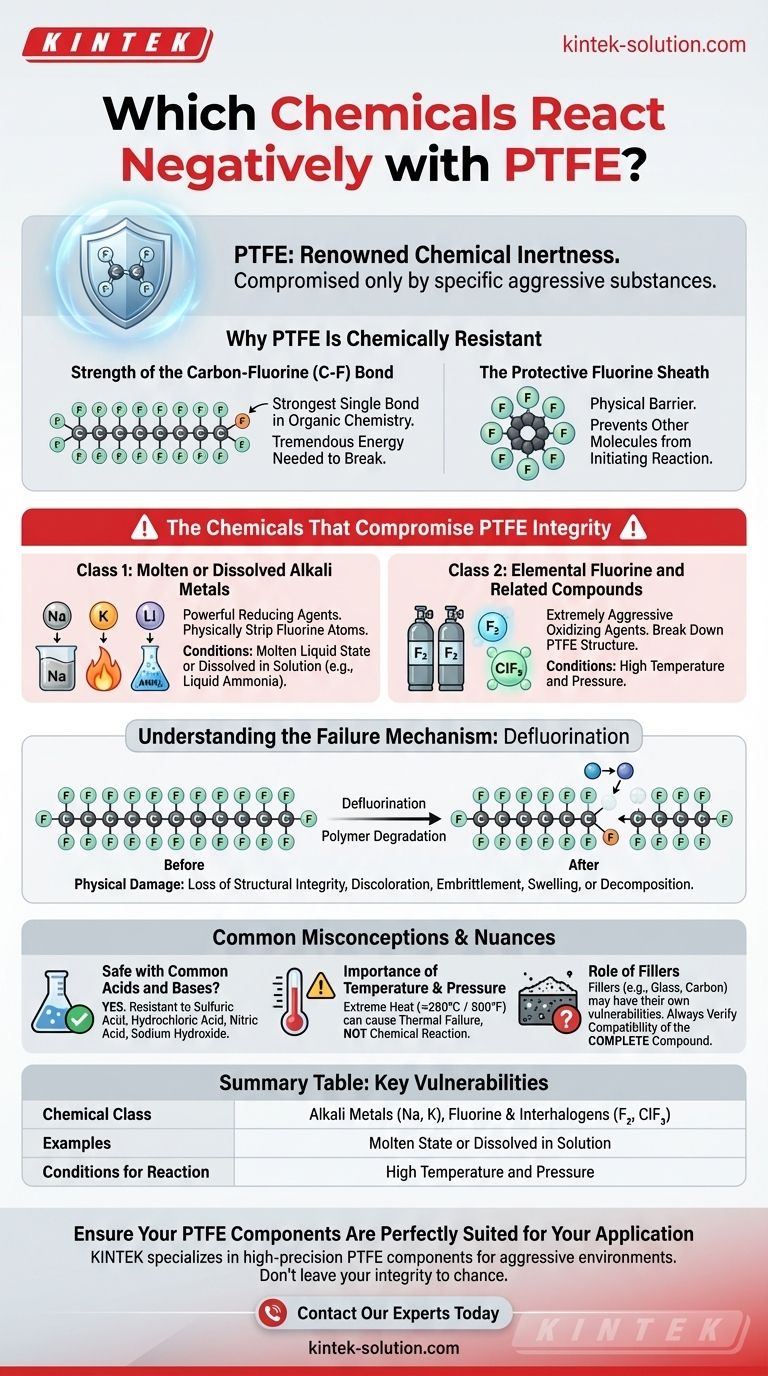
Polytetrafluoroethylene (PTFE) is renowned for its exceptional chemical inertness, but it is not invincible. While resistant to the vast majority of industrial chemicals, PTFE's integrity can be compromised by a very specific and aggressive class of substances. These include molten or dissolved alkali metals, elemental fluorine, and certain other powerful oxidizing agents under specific conditions.
The source of PTFE's legendary chemical resistance—the incredibly stable carbon-fluorine bond—is also the target of its few weaknesses. Only substances powerful enough to break this bond, such as alkali metals and elemental fluorine, can cause it to degrade.

Why PTFE Is So Chemically Resistant
To understand what damages PTFE, we must first understand why it is so remarkably durable. Its inertness stems from its unique molecular structure.
The Strength of the Carbon-Fluorine Bond
At its core, PTFE is a long chain of carbon atoms, each completely surrounded by fluorine atoms. The bond between carbon and fluorine (C-F) is one of the strongest known single bonds in organic chemistry.
This immense bond strength means it takes a tremendous amount of energy to break the molecule apart, rendering it non-reactive to common acids, bases, solvents, and oxidizers.
The Protective Fluorine Sheath
The fluorine atoms are larger than the carbon atoms they are bonded to. They effectively form a tight, continuous, and non-polar "sheath" around the vulnerable carbon backbone.
This sheath acts as a physical barrier, preventing other chemical molecules from even getting close enough to the carbon chain to initiate a reaction.
The Chemicals That Compromise PTFE Integrity
The few substances that can attack PTFE are those with the unique chemical ability to overcome its structural defenses. These reactions are rare and typically only occur in highly specialized industrial or laboratory settings.
Class 1: Molten or Dissolved Alkali Metals
This category includes elements like sodium (Na), potassium (K), and lithium (Li).
For a reaction to occur, these metals must be in their molten liquid state or dissolved in a solution (like liquid ammonia). In this state, they are powerful reducing agents that can physically strip fluorine atoms from the PTFE polymer chain.
Class 2: Elemental Fluorine and Related Compounds
Ironically, the same element that gives PTFE its strength can also be its undoing. Gaseous fluorine (F₂) and related interhalogen compounds (like chlorine trifluoride, ClF₃) are extremely aggressive oxidizing agents.
Under conditions of high temperature and pressure, these chemicals can break down the PTFE structure. This is a concern in industries that produce or work with pure fluorine gas.
Understanding the Failure Mechanism
When PTFE is attacked by one of these reactive chemicals, the process is known as defluorination.
Defluorination and Polymer Degradation
The attacking chemical essentially pulls fluorine atoms off the carbon backbone. This disrupts the protective sheath and breaks the strong C-F bonds that hold the polymer together.
The Resulting Physical Damage
As the polymer chain degrades, the material loses its structural integrity. This can manifest as discoloration (often darkening), embrittlement, swelling, or complete decomposition of the material. A seal or component made from compromised PTFE will fail.
Common Misconceptions and Nuances
It is critical to place PTFE's vulnerabilities in the proper context. For the vast majority of applications, they are not a practical concern.
Is PTFE Safe with Common Acids and Bases?
Yes. PTFE is exceptionally resistant to virtually all common and even highly concentrated acids and bases. This includes substances like sulfuric acid, hydrochloric acid, nitric acid, and sodium hydroxide.
The Importance of Temperature and Pressure
Even with compatible chemicals, extreme temperatures can be a factor. While PTFE has a high service temperature (around 260°C / 500°F), very high heat can cause it to produce toxic fumes and eventually decompose. However, this is a thermal failure, not a chemical reaction.
The Role of Fillers
Many PTFE components contain fillers like glass, carbon, or graphite to improve mechanical properties. While the PTFE itself may be inert, the filler material may have its own chemical vulnerabilities. Always verify the compatibility of the complete, filled compound.
Making the Right Choice for Your Application
Understanding these specific limitations is key to using PTFE effectively and safely.
- If your primary focus is general chemical processing, laboratory work, or food/pharma: PTFE is almost certainly a safe and highly reliable choice for seals, gaskets, tubing, and linings.
- If your application involves molten alkali metals or high-pressure fluorine gas: You must avoid standard PTFE and seek specialized materials and expert consultation for your specific conditions.
- If you are using a filled grade of PTFE: Always verify the chemical compatibility of both the PTFE and the specific filler material with your intended service media.
Knowing these distinct edge-case vulnerabilities allows you to leverage PTFE's remarkable properties with complete confidence across a wide range of demanding applications.
Summary Table:
| Chemical Class | Examples | Conditions for Reaction |
|---|---|---|
| Alkali Metals | Sodium (Na), Potassium (K) | Molten state or dissolved in solution (e.g., ammonia) |
| Fluorine & Interhalogens | Fluorine gas (F₂), Chlorine Trifluoride (ClF₃) | High temperature and pressure |
Ensure Your PTFE Components Are Perfectly Suited for Your Application
While PTFE is resistant to most chemicals, selecting the right grade and formulation is critical for aggressive environments. KINTEK specializes in manufacturing high-precision PTFE components—including seals, liners, and labware—for the semiconductor, medical, laboratory, and industrial sectors.
We prioritize precision production and offer custom fabrication from prototypes to high-volume orders, ensuring your components meet exact chemical compatibility and performance requirements.
Don't leave your application's integrity to chance. Contact our experts today for a consultation on your specific needs.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Customizable PTFE Rods for Advanced Industrial Applications
People Also Ask
- What is the working temperature range of PTFE? Master Extreme Heat and Cryogenic Applications
- What is PTFE commonly known as and what are its unique properties? Unlock Unmatched Chemical & Thermal Resistance
- What are the material advantages of machining Teflon? Unlock Unmatched Chemical & Thermal Resistance
- Why is PTFE suitable for cryogenic or high-temperature applications? Unmatched Thermal Stability from -450°F to 500°F
- What are the base characteristics of PTFE? Unlocking Extreme Performance in Friction, Temperature, and Chemical Resistance



















