The unique characteristic of Polytetrafluoroethylene (PTFE) that prevents geckos from sticking to it is its extremely low surface energy. This property, a result of its unique molecular structure, makes its "anti-adhesive" and "low friction" characteristics so pronounced that the intermolecular forces a gecko relies on for adhesion are too weak to form a bond.
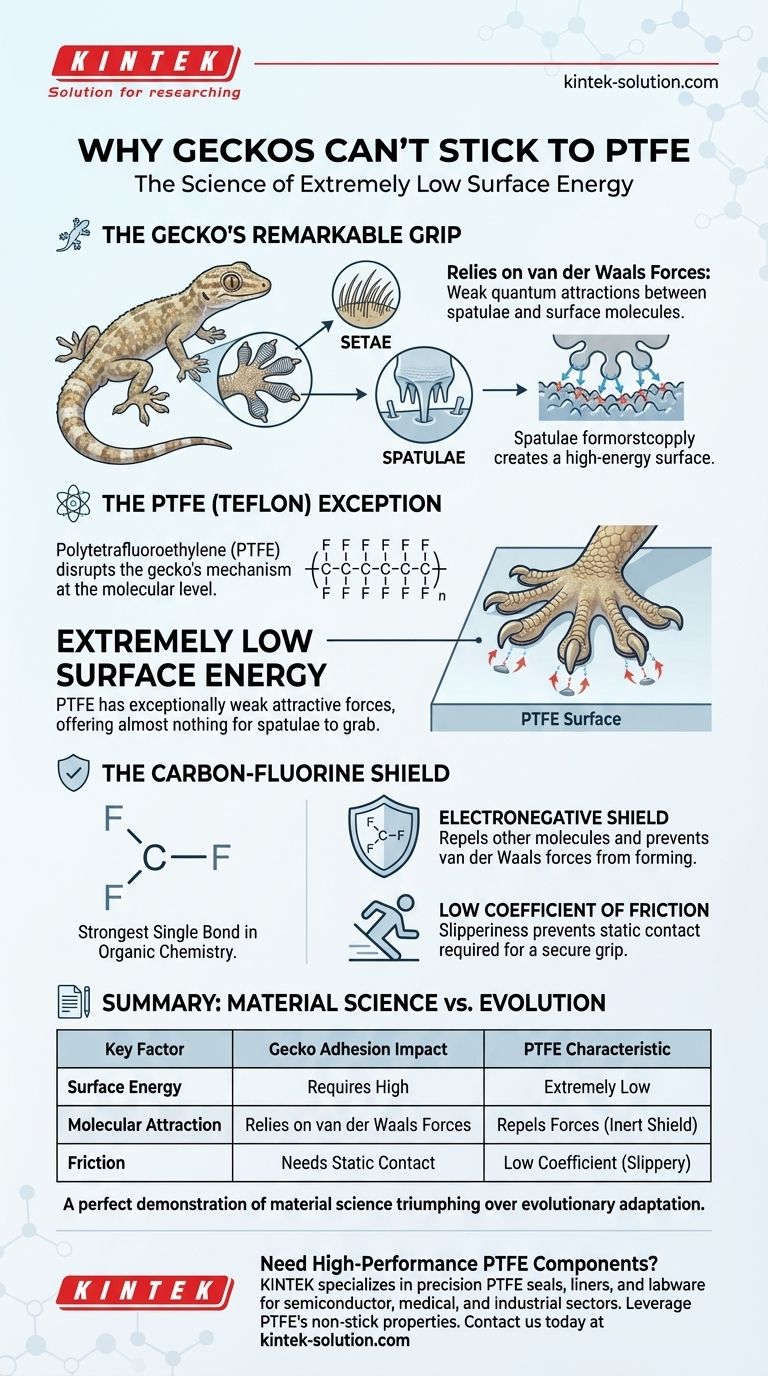
A gecko's remarkable climbing ability is not due to suction or a chemical adhesive, but to the collective power of millions of weak intermolecular attractions. PTFE is the exception because its surface is so chemically inert and non-polar that it simply doesn't offer enough attraction for the gecko's foot to get a grip.

First, Understand the Gecko's Grip
To understand why PTFE works, you must first understand the gecko's mechanism. It's a marvel of physics, not biology alone.
The Power of van der Waals Forces
A gecko's feet are covered in millions of microscopic, hair-like structures called setae. Each seta branches out into hundreds of even smaller tips called spatulae.
This structure creates an enormous surface area. When the gecko places its foot on a surface, the close proximity between the spatulae and the surface molecules allows a weak quantum force, known as the van der Waals force, to form.
While a single van der Waals force is incredibly weak, the sum of these interactions across millions of spatulae creates the powerful adhesion that allows a gecko to support its body weight on a vertical wall or even hang from a ceiling.
Why PTFE Is the Exception
PTFE, commonly known by the brand name Teflon, systematically dismantles the gecko's adhesion mechanism at the molecular level.
The Critical Role of Low Surface Energy
Every material has a property called surface energy, which is a measure of the excess energy at its surface compared to its bulk. Materials with high surface energy readily attract other molecules.
PTFE has one of the lowest surface energies of any known solid. Its surface has exceptionally weak forces of attraction, meaning there is almost nothing for the gecko's spatulae to "grab" onto. The van der Waals forces simply cannot be established with enough strength to create a bond.
The Carbon-Fluorine Bond Shield
The source of this low surface energy is PTFE's chemical structure. It consists of a long chain of carbon atoms completely shielded by a helix of fluorine atoms.
The carbon-fluorine bond is one of the strongest single bonds in organic chemistry. This makes the molecule incredibly stable and non-reactive. The fluorine atoms create an "electronegative shield" that repels nearly all other molecules, preventing the intermolecular attraction necessary for adhesion.
A Low Coefficient of Friction
The references are correct to highlight PTFE's low coefficient of friction. This "slipperiness" is a direct consequence of its low surface energy. Because other molecules don't stick to it, they slide off effortlessly. For a gecko, this means its spatulae can't maintain the static contact required for a secure grip.
Why Other "Slippery" Materials Don't Work
This raises a key question: why can geckos stick to smooth glass but not PTFE? The answer lies in the difference between macroscopic smoothness and molecular interaction.
The Molecular-Level Difference
A material like polished glass or even a surface with a thin layer of oil might feel slippery to us. However, at the microscopic level where the spatulae operate, these surfaces are still chemically active.
Glass, for instance, has a high surface energy and offers plenty of opportunities for van der Waals forces to form. PTFE's resistance is not just a surface finish; it is an inherent property of its molecular structure.
Making the Right Choice for Your Understanding
To grasp this fascinating interaction, it's helpful to separate the biological mechanism from the material science principle.
- If your primary focus is the gecko: The key takeaway is that its adhesion relies on maximizing surface area to aggregate billions of weak van der Waals forces.
- If your primary focus is PTFE: The key takeaway is that its exceptionally stable carbon-fluorine bonds create an inert, low-energy surface that repels other molecules.
Ultimately, the battle between a gecko's foot and a PTFE surface is a perfect demonstration of material science triumphing over a brilliant evolutionary adaptation.
Summary Table:
| Key Factor | Why It Matters for Gecko Adhesion |
|---|---|
| Low Surface Energy | PTFE's surface has weak attraction forces, preventing van der Waals bonds from forming. |
| Carbon-Fluorine Bond Shield | Strong, stable bonds create an electronegative shield that repels other molecules. |
| Low Coefficient of Friction | PTFE's slipperiness prevents static contact needed for a gecko's secure grip. |
Need High-Performance PTFE Components?
KINTEK specializes in manufacturing precision PTFE seals, liners, and labware for the semiconductor, medical, and industrial sectors. Our custom fabrication—from prototypes to high-volume orders—ensures your components leverage PTFE's unique non-stick and low-friction properties.
Contact us today to discuss your specific requirements!
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
People Also Ask
- What finishing techniques are effective for machined Teflon parts? Achieve Functional Performance and Dimensional Stability
- What are the main applications of PTFE type Teflon? Unlock Its Versatility for Your Industry
- What industrial benefits do PTFE-machined parts offer? Achieve Peak Performance in Demanding Applications
- What factors should be considered when choosing between Nylon and PTFE? Select the Right Material for Your Application
- What chemical processing applications involve PTFE-machined parts? Essential Components for Corrosive & High-Purity Systems



















