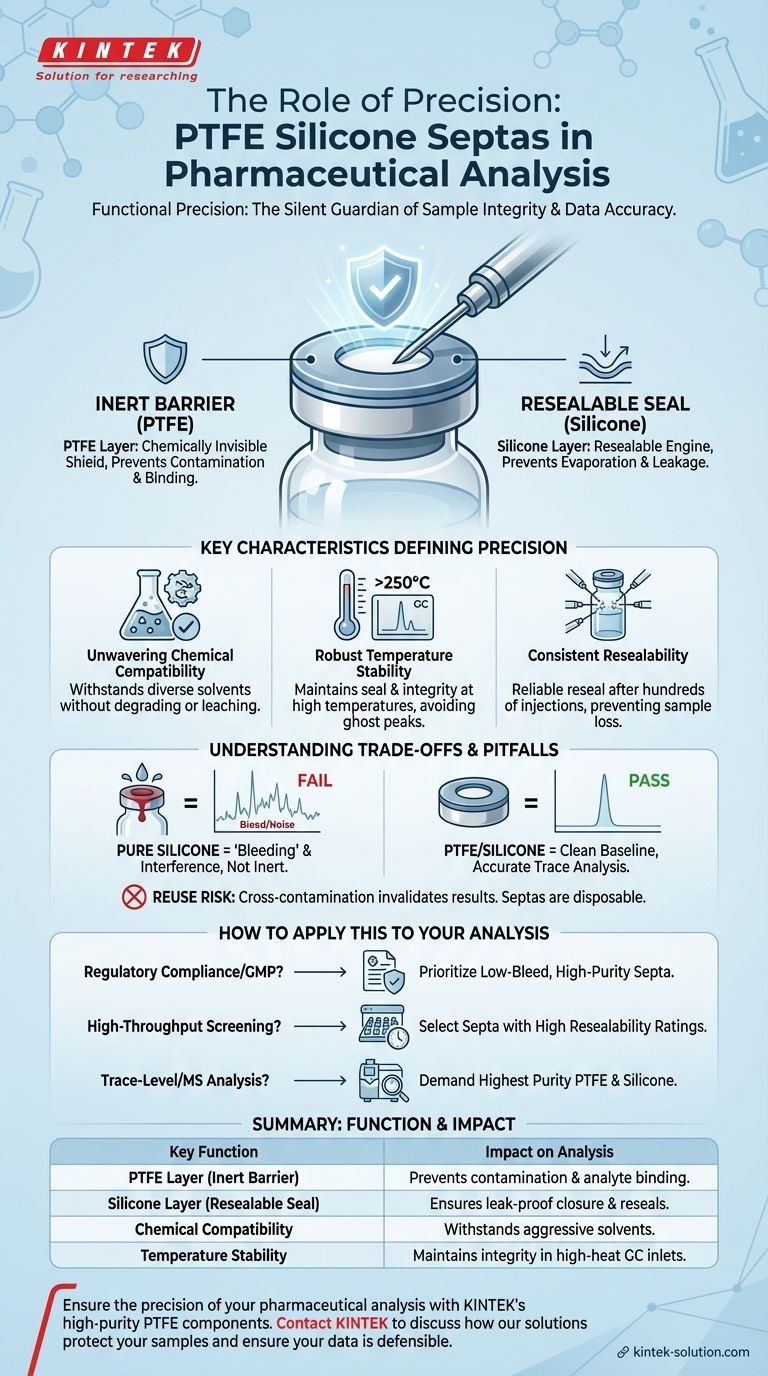
In pharmaceutical analysis, the "precision" of a PTFE silicone septa is not about its manufacturing dimensions, but its functional ability to guarantee the precision of your analytical results. These septa provide an inert, leak-free, and resealable barrier that protects the sample from contamination and evaporation. This function is the bedrock of accurate, repeatable, and compliant data in highly regulated environments.
The role of a PTFE silicone septa is to act as a silent guardian of sample integrity. Its precision lies in its consistent performance—creating a perfect seal and remaining chemically inert—which directly translates into the precision and reliability of the final analytical measurement.

The Anatomy of Analytical Precision
A PTFE silicone septa is a composite component designed to solve two distinct problems simultaneously. Its design directly impacts the integrity of data generated by sensitive instruments like HPLC (High-Performance Liquid Chromatography) and GC-MS (Gas Chromatography-Mass Spectrometry).
The PTFE Layer: Your Inert Chemical Shield
The layer of PTFE (polytetrafluoroethylene) is the only part of the septa that faces the sample. Its primary job is to be chemically invisible.
Because PTFE is almost universally non-reactive, it does not leach contaminants into the sample or bind with the analytes you are trying to measure. This ensures the chemical composition of your sample remains unchanged from the vial to the instrument.
The Silicone Layer: The Engine of a Resealable Seal
Behind the PTFE shield is a thicker layer of high-purity silicone. This material provides the mechanical functionality of the septa.
Silicone's elasticity allows it to form a tight, leak-proof seal against the rim of the vial. Crucially, it also allows the septa to reseal after being punctured by an autosampler needle, preventing sample evaporation and protecting against atmospheric contamination over the course of an analytical sequence.
Key Characteristics That Define Precision
The performance of a septa is defined by several key characteristics. A failure in any one of these areas can compromise an entire batch of analyses.
Unwavering Chemical Compatibility
Pharmaceutical solvents can range from acidic to alkaline to aggressive organic mixtures. A high-precision septa must withstand all of them without degrading, swelling, or releasing impurities. This is a non-negotiable property provided by the PTFE layer.
Robust Temperature Stability
In Gas Chromatography (GC), samples are injected into a heated inlet port, often exceeding 250°C. The septa must maintain its seal and chemical integrity at these temperatures. A low-quality septa can degrade, introducing "ghost peaks" and system contamination that can take hours to clean.
Consistent Resealability
Modern laboratories rely on autosamplers that may perform hundreds of injections from a single tray of vials. A septa's ability to reseal reliably after each puncture is essential for maintaining consistent results from the first injection to the last. Failure here leads to sample evaporation, concentrating the non-volatile components and corrupting all subsequent data.
Understanding the Trade-offs and Pitfalls
Choosing the right septa is a critical decision, and misunderstanding its function can lead to significant issues.
Why "Pure Silicone" Fails in Demanding Analyses
While less expensive, septa made of only silicone are unsuitable for most pharmaceutical work. Silicone is not chemically inert and is prone to "bleeding"—leaching siloxane compounds that cause significant background noise and interfere with trace analysis, especially in GC-MS.
The True Cost of a Failed Seal
A leaking or contaminated septa doesn't just waste a single sample. It can invalidate an entire analytical run, compromise expensive reference standards, and lead to hours of troubleshooting and instrument downtime. In a regulated setting, it can trigger costly out-of-specification investigations.
Reusability vs. Carryover Risk
The term "reusability" in the context of septa primarily refers to its ability to withstand multiple punctures within a single analytical sequence. However, septa are fundamentally disposable. Reusing a vial and septa for a different sample creates an unacceptable risk of cross-contamination, which can invalidate results.
How to Apply This to Your Analysis
Your analytical goal should dictate your choice of septa.
- If your primary focus is regulatory compliance and GMP: Prioritize septas with documented specifications for low bleed and high purity to ensure your data is defensible.
- If your primary focus is high-throughput screening: Select septa specifically rated for high resealability to ensure consistency across hundreds or thousands of automated injections.
- If your primary focus is trace-level analysis or mass spectrometry: Demand the highest-purity PTFE and silicone to eliminate any risk of background signals or ghost peaks interfering with your results.
Ultimately, the septa is a small but critical component that provides the foundation of trust for your entire analytical workflow.
Summary Table:
| Key Function | Impact on Analysis |
|---|---|
| PTFE Layer (Inert Barrier) | Prevents sample contamination and analyte binding. |
| Silicone Layer (Resealable Seal) | Ensures leak-proof closure and reseals after needle puncture. |
| Chemical Compatibility | Withstands aggressive solvents without degrading. |
| Temperature Stability | Maintains integrity in high-heat GC inlets (>250°C). |
Ensure the precision of your pharmaceutical analysis with KINTEK's high-purity PTFE components.
Your analytical results are only as reliable as the components protecting your samples. KINTEK specializes in manufacturing precision PTFE seals, septa, and labware for the semiconductor, medical, and laboratory industries. We understand the critical need for chemically inert, leak-free barriers that guarantee sample integrity from the first injection to the last in demanding HPLC and GC-MS workflows.
Whether you require standard parts or custom-fabricated prototypes for high-volume GMP production, our commitment to precision manufacturing delivers the consistency and purity your sensitive analyses demand. Don't let a minor component compromise your entire batch.
Contact KINTEK today to discuss how our PTFE solutions can protect your samples and ensure your data is defensible.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Customizable PTFE Seals Filter Holders for Versatile Applications
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
People Also Ask
- Why are PTFE and silicone rubber chosen as materials for HPLC septa? Ensure Sample Integrity and Reliable Sealing
- What are the key components of the Low-Bleed PTFE/Silicone headspace septa and their functions? Ensure Sample Integrity in GC Analysis
- What are the benefits of selecting the appropriate PTFE-coated septum for chromatography? Ensure Accurate & Reproducible Results
- What are the key applications of the PTFE bottle? Ensure Chemical Safety and Sample Purity
- What are the advantages of using PTFE lined caps? Superior Sealing for Volatile Liquids & High-Temp Storage
- What are the best practices for using PTFE-lined caps in chromatography? Ensure Data Integrity and Prevent Contamination
- What are the benefits of using high-performance materials in chemical laboratories? Ensure Purity and Reliability
- What are PTFE ferromagnetic support discs composed of? A Dual-Material Design for Superior Grinding & Polishing



















