At its core, the unique chemical makeup of Polytetrafluoroethylene (PTFE) is remarkably simple. It is a synthetic fluoropolymer, a high-molecular-weight compound consisting solely of carbon and fluorine. This specific combination and the powerful bond between these two elements are the direct source of its famous properties, including its extreme chemical resistance and exceptionally low friction.
The key to understanding PTFE is recognizing that its entire performance profile stems from the strength and stability of the carbon-fluorine bond. This bond creates a chemically inert and non-polar molecular structure, which in turn gives the material its non-stick, non-reactive, and dielectric characteristics.

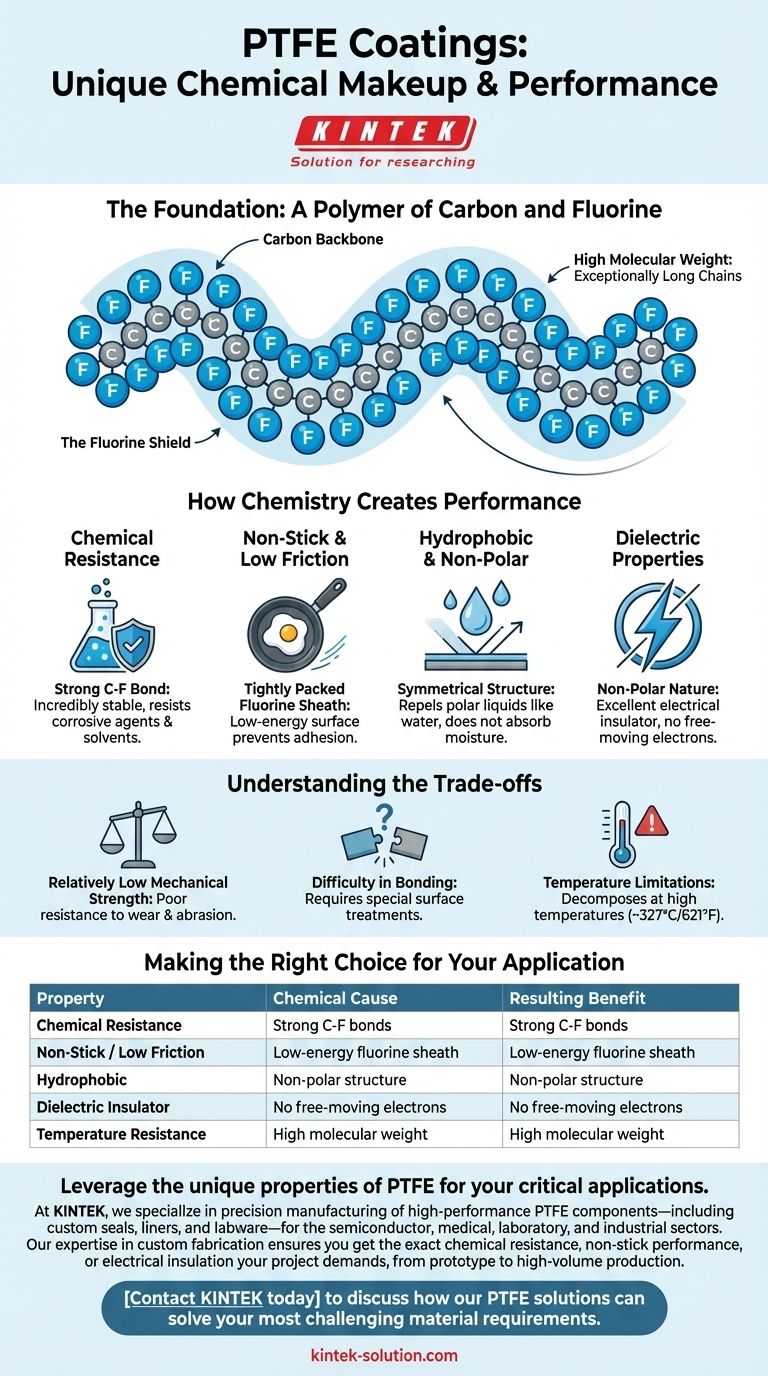
The Foundation: A Polymer of Carbon and Fluorine
PTFE's structure is the direct cause of its function. Understanding this simple but powerful atomic arrangement is the first step to appreciating why it behaves the way it does.
The Carbon Backbone
The molecule is built upon a long, linear chain of carbon atoms. This forms the structural "backbone" of the polymer.
The Fluorine Shield
Each carbon atom in the chain is bonded to two fluorine atoms. These fluorine atoms are relatively large and tightly packed, forming a protective, non-reactive "sheath" that completely encases the carbon backbone.
High Molecular Weight
PTFE molecules are exceptionally long, giving the material a high molecular weight. This contributes to its physical stability, strength, and high melting point compared to simpler, smaller molecules.
How Chemistry Creates Performance
The properties that make PTFE so valuable in industrial, medical, and consumer applications are not accidental. They are a direct consequence of its atomic structure.
The Strength of the Carbon-Fluorine Bond
The bond between carbon and fluorine is one of the strongest known in organic chemistry. This exceptional strength makes the molecule incredibly stable and non-reactive. It is difficult for other chemicals to break these bonds, which is why PTFE is resistant to nearly all corrosive agents and solvents.
The "Tightly Packed" Fluorine Sheath
The outer layer of fluorine atoms creates a surface with very low energy. Because the fluorine atoms hold their electrons so tightly, there is very little intermolecular attraction. This is the source of PTFE's famous non-stick and low-friction (low coefficient of friction) properties. Other substances simply cannot find a way to "grab onto" the surface.
Hydrophobic and Non-Polar Nature
The symmetrical arrangement of the fluorine atoms around the carbon chain creates a non-polar molecule. This structure repels polar liquids like water, making PTFE highly hydrophobic (water-repellent) and ensuring it does not absorb moisture.
Dielectric Properties
The same non-polar nature means there are no free-moving electrons within the molecular structure. This makes PTFE an outstanding electrical insulator, or dielectric material, as it cannot conduct an electrical current.
Understanding the Trade-offs
No material is perfect, and the same chemistry that gives PTFE its strengths also creates its limitations. Acknowledging these trade-offs is critical for proper application.
Relatively Low Mechanical Strength
The weak forces between separate PTFE molecules, which contribute to its low friction, also mean it has poor resistance to wear, creep, and abrasion. It is a relatively soft material that can be easily scratched.
Difficulty in Bonding
Its non-stick surface is an advantage in a frying pan but a challenge in manufacturing. Bonding PTFE to other materials is notoriously difficult and requires special surface treatments like chemical etching to create a viable bond.
Temperature Limitations
While PTFE has a high service temperature, it undergoes a phase transition around 327°C (621°F) and begins to decompose at higher temperatures. Overheating PTFE can release potentially toxic fumes, a critical safety consideration in its application and use.
Making the Right Choice for Your Application
Selecting PTFE should be a deliberate choice based on its specific chemical advantages.
- If your primary focus is chemical resistance: PTFE is an almost unmatched choice for seals, gaskets, and linings in harsh chemical processing environments due to its inert C-F bonds.
- If your primary focus is low friction or a non-stick surface: Its low-energy fluorine sheath makes it ideal for release coatings, medical catheters, and low-friction bearings.
- If your primary focus is electrical insulation: PTFE's non-polar structure makes it a top-tier material for high-frequency cables and printed circuit boards.
Ultimately, PTFE's unique value is a direct result of its simple, stable, and powerful chemical composition.
Summary Table:
| Property | Chemical Cause | Resulting Benefit |
|---|---|---|
| Chemical Resistance | Strong C-F bonds are hard to break | Resists nearly all corrosive agents and solvents |
| Non-Stick / Low Friction | Tightly packed fluorine sheath creates a low-energy surface | Prevents substances from adhering; ideal for release coatings |
| Hydrophobic | Non-polar molecular structure | Repels water and does not absorb moisture |
| Dielectric Insulator | No free-moving electrons in the non-polar structure | Excellent electrical insulator for high-frequency applications |
| Temperature Resistance | High molecular weight and stable bonds | High melting point and service temperature (up to ~327°C/621°F) |
Leverage the unique properties of PTFE for your critical applications.
At KINTEK, we specialize in precision manufacturing of high-performance PTFE components—including custom seals, liners, and labware—for the semiconductor, medical, laboratory, and industrial sectors. Our expertise in custom fabrication ensures you get the exact chemical resistance, non-stick performance, or electrical insulation your project demands, from prototype to high-volume production.
Contact KINTEK today to discuss how our PTFE solutions can solve your most challenging material requirements.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
People Also Ask
- What design considerations are important for custom PTFE parts? Design for Performance & Reliability
- What challenges arise when machining PTFE (Teflon)? Overcome Softness, Heat, and Instability
- What factors should be considered when choosing between Nylon and PTFE? Select the Right Material for Your Application
- What are the main applications of PTFE type Teflon? Unlock Its Versatility for Your Industry
- What industrial benefits do PTFE-machined parts offer? Achieve Peak Performance in Demanding Applications



















