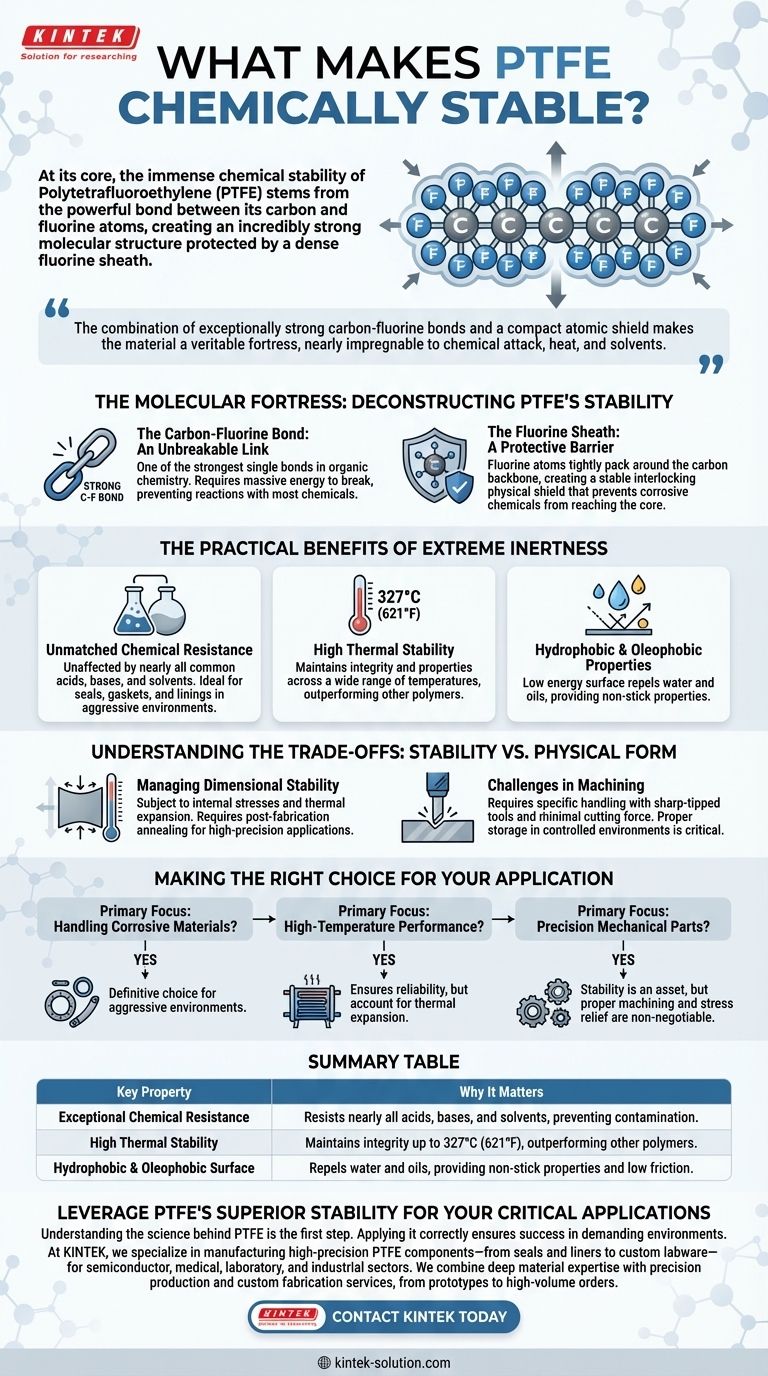
At its core, the immense chemical stability of Polytetrafluoroethylene (PTFE) stems from the powerful bond between its carbon and fluorine atoms. This creates an incredibly strong molecular structure that is further protected by a dense, interlocking sheath of fluorine atoms, rendering it almost completely inert.
The key to PTFE's resilience is its molecular architecture. The combination of exceptionally strong carbon-fluorine bonds and a compact atomic shield makes the material a veritable fortress, nearly impregnable to chemical attack, heat, and solvents.

The Molecular Fortress: Deconstructing PTFE's Stability
To truly understand why PTFE is so reliable in harsh environments, we must look at its structure on a microscopic level. It's a simple polymer, but its design is uniquely robust.
The Carbon-Fluorine Bond: An Unbreakable Link
The bond between a carbon atom and a fluorine atom is one of the strongest single bonds in organic chemistry.
This exceptional bond strength means a massive amount of energy is required to break it, which is why PTFE does not react with most other chemicals.
The Fluorine Sheath: A Protective Barrier
The carbon atoms in PTFE form a long chain, or "backbone." This backbone is completely surrounded by fluorine atoms.
These fluorine atoms are relatively large and packed tightly together, creating a stable, interlocking physical shield. This sheath prevents corrosive chemicals from even reaching the vulnerable carbon backbone.
The Practical Benefits of Extreme Inertness
This molecular stability translates directly into the properties that make PTFE invaluable across numerous industries, from aerospace to pharmaceuticals.
Unmatched Chemical Resistance
PTFE is unaffected by nearly all common acids, bases, and solvents.
This allows it to be used for seals, gaskets, and linings in equipment that handles highly corrosive or pure substances without risk of degradation or contamination.
High Thermal Stability
The same bond strength that provides chemical resistance also gives PTFE a very high melting point of 327°C (621°F).
It maintains its integrity and properties across a wide range of temperatures where other polymers would fail.
Hydrophobic and Oleophobic Properties
The high concentration of fluorine atoms creates a surface with very low energy.
This makes PTFE highly repellent to both water (hydrophobic) and oils (oleophobic), which is the basis for its famous non-stick properties.
Understanding the Trade-offs: Stability vs. Physical Form
While chemically and thermally stable, PTFE's unique structure presents specific challenges in fabrication and application that must be managed.
Managing Dimensional Stability
PTFE can be subject to internal stresses from the manufacturing process and can expand or contract significantly with temperature changes.
For high-precision applications, parts often require post-fabrication annealing (a controlled heating and cooling process) to relieve these stresses and ensure dimensional reliability.
Challenges in Machining
The material's properties require specific handling during fabrication to maintain its integrity.
Using sharp-tipped tools with minimal cutting force is essential for precision machining. Storing the material in controlled environments away from high heat or humidity is also critical to preserving its form.
Making the Right Choice for Your Application
Understanding these properties allows you to leverage PTFE's strengths effectively.
- If your primary focus is handling corrosive materials: Its unparalleled chemical inertness makes it the definitive choice for gaskets, linings, and tubing in aggressive environments.
- If your primary focus is high-temperature performance: Its high melting point ensures reliability, but you must account for thermal expansion in your design to maintain tight tolerances.
- If your primary focus is precision mechanical parts: Its stability is a major asset, but proper machining and post-fabrication stress relief are non-negotiable to achieve and maintain dimensional accuracy.
By appreciating both its molecular strength and its physical handling requirements, you can deploy PTFE with confidence in the most demanding applications.
Summary Table:
| Key Property | Why It Matters |
|---|---|
| Exceptional Chemical Resistance | Resists nearly all acids, bases, and solvents, preventing contamination. |
| High Thermal Stability | Maintains integrity up to 327°C (621°F), outperforming other polymers. |
| Hydrophobic & Oleophobic Surface | Repels water and oils, providing non-stick properties and low friction. |
Leverage PTFE's Superior Stability for Your Critical Applications
Understanding the science behind PTFE is the first step. Applying it correctly is what ensures success in demanding environments. At KINTEK, we specialize in manufacturing high-precision PTFE components—from seals and liners to custom labware—for the semiconductor, medical, laboratory, and industrial sectors.
We combine deep material expertise with precision production and custom fabrication services, from prototypes to high-volume orders, ensuring your components deliver unmatched chemical resistance and thermal stability.
Ready to enhance your project's reliability? Contact KINTEK today to discuss your specific requirements and discover how our PTFE solutions can solve your most challenging problems.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Customizable PTFE Seals Filter Holders for Versatile Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- What industrial applications does PTFE have? Unlock Performance in Extreme Environments
- What material is the PTFE bottle made from? Discover the Benefits of 100% Virgin PTFE
- What are the primary applications of Teflon? Leverage Its Unique Properties for Your Industry
- What are the common characteristics of Teflon? Unlocking Extreme Chemical and Thermal Resistance
- What makes the PTFE bottle durable? Unmatched Chemical & Thermal Stability for Demanding Applications



















