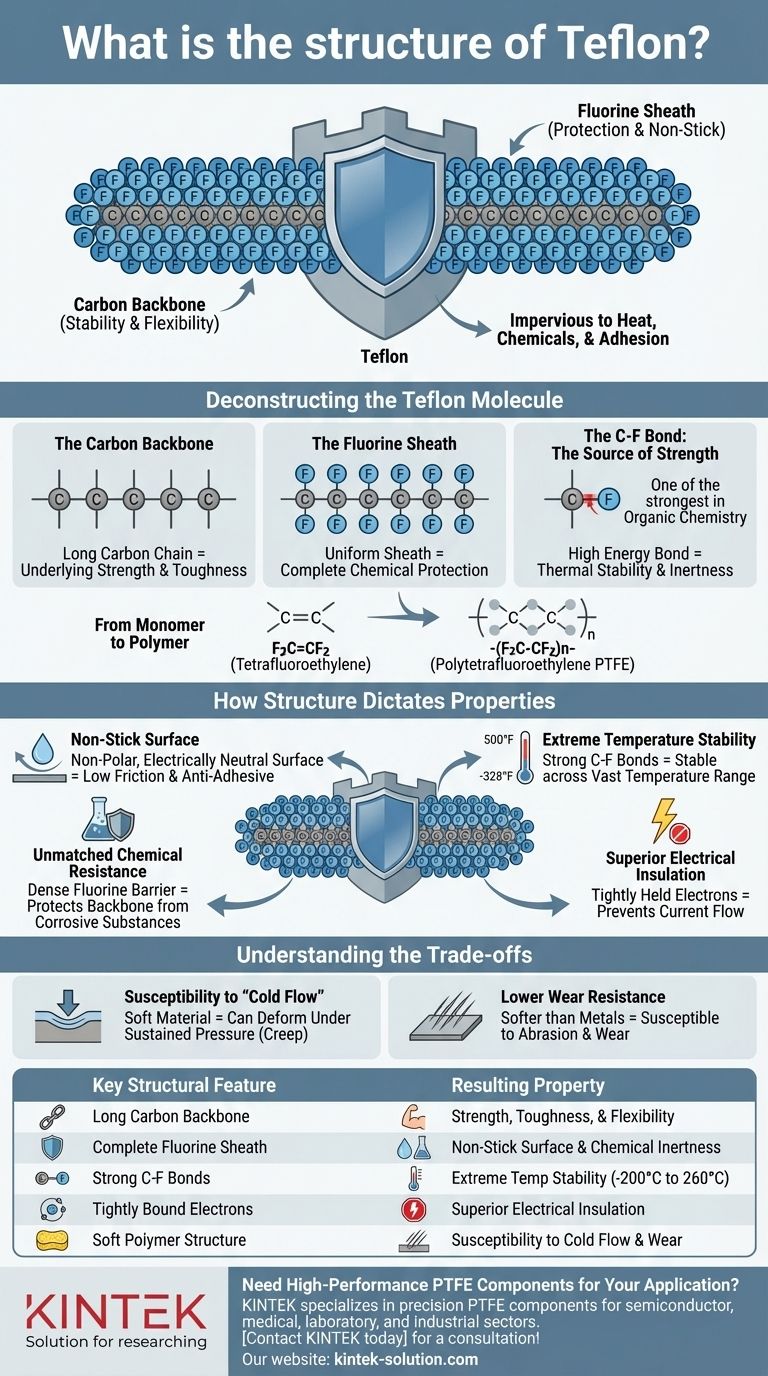
At its core, Teflon is a polymer known scientifically as polytetrafluoroethylene (PTFE). Its structure consists of a long, repeating chain of carbon atoms, where each carbon is bonded to two fluorine atoms. This simple but incredibly stable molecular arrangement, represented by the formula (C2F4)n, is the source of all of Teflon's well-known properties.
The key to understanding Teflon is to visualize its structure not just as a chain, but as a fortress. Its stable carbon backbone is completely shielded by a tightly packed, non-reactive sheath of fluorine atoms, making it almost impervious to heat, chemicals, and adhesion.

Deconstructing the Teflon Molecule
To appreciate why Teflon behaves the way it does, we must examine its constituent parts. The elegance of its design lies in the simplicity and strength of its chemical bonds.
The Carbon Backbone
The foundation of the Teflon molecule is a long, continuous chain of carbon atoms (-C-C-C-). This polymer backbone gives the material its underlying strength, toughness, and flexibility.
The Fluorine Sheath
The defining feature of Teflon is that every available bonding spot on the carbon backbone is occupied by a fluorine atom. This creates a uniform, protective "sheath" of fluorine that completely encases the carbon chain.
The C-F Bond: The Source of Strength
The bond between carbon and fluorine (C-F) is one of the strongest single bonds in organic chemistry. It requires a tremendous amount of energy to break, which is the fundamental reason for Teflon's remarkable thermal stability and chemical inertness.
From Monomer to Polymer
Teflon is created through the polymerization of tetrafluoroethylene (F2C=CF2) molecules. In this process, the double bond in the monomer breaks, allowing them to link together end-to-end, forming the long -(F2C-CF2)n- chain that constitutes the final material.
How Structure Dictates Teflon's Famous Properties
Every key characteristic of Teflon is a direct consequence of its tightly bonded, fluorine-shielded structure. The molecule's architecture dictates its function.
The Secret to its "Non-Stick" Surface
The fluorine atoms on the surface create an electrically neutral, non-polar sheath with extremely weak intermolecular forces. Because other substances have nothing to "grab" onto, they slide right off, resulting in an exceptionally low coefficient of friction and its famous anti-adhesive quality.
Unmatched Chemical Resistance
The dense fluorine sheath acts as a formidable barrier, protecting the chemically vulnerable carbon backbone from attack. The C-F bonds themselves are so strong that very few chemicals have the energy to react with them, making Teflon resistant to nearly all corrosive substances.
Extreme Temperature Stability
Because the C-F bonds are so powerful, it takes a significant amount of thermal energy to disrupt them. This allows Teflon to remain stable and functional across a vast temperature range, from a cryogenic -328°F (-200°C) up to 500°F (260°C).
Superior Electrical Insulation
The electrons within the C-F bonds are held very tightly by the fluorine atoms. This lack of mobile electrons makes Teflon an outstanding electrical insulator, preventing the flow of current.
Understanding the Trade-offs
While its properties are exceptional, no material is perfect. Understanding the limitations inherent in Teflon's structure is crucial for its proper application.
Susceptibility to "Cold Flow"
PTFE is a relatively soft material. Under sustained pressure, especially at room temperature, it can slowly deform or "creep." This means it is not typically used for high-load structural components without reinforcement.
Lower Wear Resistance
Compared to harder materials like metals or certain engineering plastics, Teflon's surface can be susceptible to abrasion. Its low-friction properties can be compromised if the surface is scratched or worn away.
Making the Right Choice for Your Goal
The decision to use Teflon should be based on a clear understanding of how its molecular structure serves your primary objective.
- If your primary focus is chemical inertness: Teflon's fluorine-shielded backbone makes it the default choice for seals, linings, and components exposed to aggressive chemicals.
- If your primary focus is low friction: The non-polar fluorine surface provides one of the lowest friction coefficients of any solid, making it ideal for non-stick coatings and self-lubricating bearings.
- If your primary focus is temperature stability: The immense strength of the C-F bond ensures material integrity in both high-heat and cryogenic environments where most other polymers would degrade.
By linking Teflon's performance back to its simple and robust molecular structure, you can confidently determine where its unique strengths are best applied.
Summary Table:
| Key Structural Feature | Resulting Property |
|---|---|
| Long Carbon Backbone | Strength, Toughness, and Flexibility |
| Complete Fluorine Sheath | Non-Stick Surface and Chemical Inertness |
| Strong Carbon-Fluorine (C-F) Bonds | Extreme Temperature Stability (-200°C to 260°C) |
| Tightly Bound Electrons | Superior Electrical Insulation |
| Soft Polymer Structure | Susceptibility to Cold Flow and Lower Wear Resistance |
Need High-Performance PTFE Components for Your Application?
Understanding Teflon's structure is the first step. Applying it effectively is the next. KINTEK specializes in manufacturing precision PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors.
We leverage PTFE's unique properties to create solutions that offer:
- Unmatched Chemical Resistance for harsh environments.
- Reliable Non-Stick Performance and low friction.
- Stable Operation across extreme temperatures.
From custom prototypes to high-volume production, we ensure precision and quality. Let's discuss how our expertise in PTFE can solve your specific challenges.
Contact KINTEK today for a consultation!
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
People Also Ask
- What industrial applications utilize expanded PTFE? Sealing, Filtration & Insulation Solutions
- What is the molecular structure of PTFE? The Key to Its Unmatched Chemical & Thermal Resistance
- What are the key heat resistance properties of PTFE? Master Extreme Temperature Applications
- What is expanded PTFE and how does it differ from conventional PTFE? A Guide to Material Selection
- How does material selection impact PCB manufacturing and cost? Optimize Performance and Budget
- How is ePTFE structured and what are its properties? Unlock Advanced Performance with Microporous PTFE
- How does Teflon's chemical resistance benefit industrial applications? Ensure Purity and Prevent Corrosion
- When and by whom was PTFE discovered? A Tale of Accidental Innovation



















