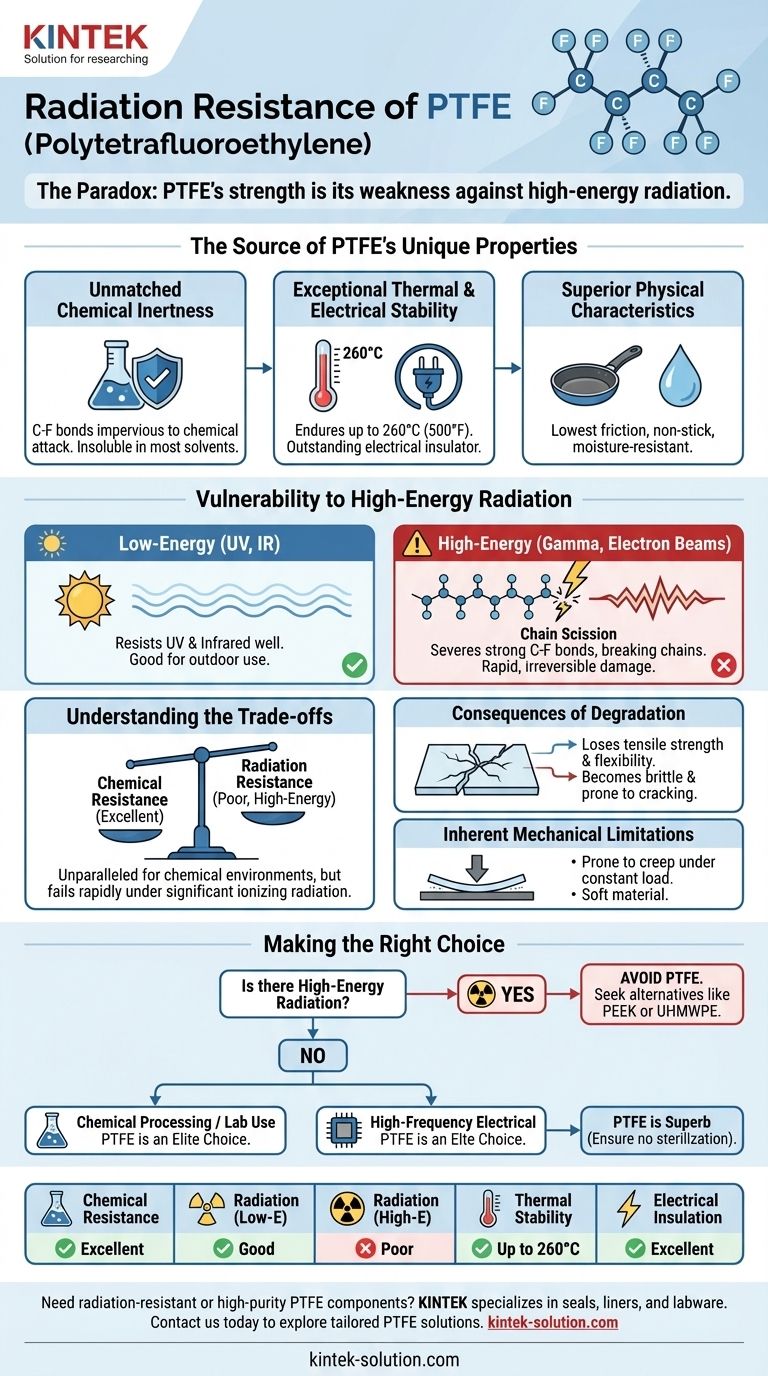
To be precise, Polytetrafluoroethylene (PTFE) has poor resistance to high-energy radiation, such as gamma rays or electron beams, which cause its molecular structure to break down. While it is highly resistant to low-energy radiation like UV, its strong carbon-fluorine bonds are susceptible to scission when exposed to ionizing radiation, leading to a significant loss of mechanical properties. The provided references do not contain specific data for modified PTFE (mPTFE), but the fundamental vulnerability of the polymer's backbone remains a critical consideration.
The core issue is a paradox: the exceptionally strong carbon-fluorine bonds that give PTFE its remarkable chemical inertness are the very same structures that, when broken by high-energy radiation, lead to rapid and irreversible degradation of the material.

The Source of PTFE's Unique Properties
To understand PTFE's radiation vulnerability, we must first appreciate the molecular structure that gives it its most valued characteristics. The entire material is built on a foundation of incredibly stable bonds.
### Unmatched Chemical Inertness
The strength of the carbon-fluorine (C-F) bonds makes PTFE virtually impervious to chemical attack.
It is insoluble in all known solvents and is only attacked by a few exotic substances like molten alkali metals and fluorine at high temperatures. This makes it an elite choice for handling aggressive acids, bases, and organic solvents.
### Exceptional Thermal and Electrical Stability
PTFE can endure continuous service temperatures up to 260°C (500°F) without significant degradation.
Its structure also makes it an outstanding electrical insulator with excellent dielectric properties, which is why it is frequently used in high-frequency applications like coaxial cables and circuit boards.
### Superior Physical Characteristics
PTFE possesses the lowest coefficient of friction of any known solid, giving it its famous non-stick quality. It is also non-adhesive, moisture-resistant, and weatherable.
The Vulnerability to High-Energy Radiation
The stability that makes PTFE so robust in chemical and thermal environments becomes its primary weakness when faced with high-energy ionizing radiation.
### How High-Energy Radiation Causes Damage
High-energy radiation, like gamma rays, carries enough power to sever the strong carbon-fluorine bonds.
This process, known as chain scission, breaks the long polymer chains that give the material its strength and structure. The material does not have an effective mechanism to dissipate this energy safely.
### The Critical Distinction: Low vs. High Energy
PTFE easily resists low-energy radiation such as UV and infrared (IR). This is why it performs well in outdoor applications.
However, it is the high-energy, ionizing radiation used in applications like medical sterilization or found in nuclear environments that causes severe and rapid damage.
### The Consequences of Degradation
When the polymer chains are broken, the material's integrity is compromised.
PTFE loses its tensile strength and flexibility, becoming brittle and prone to cracking. This degradation renders it unusable for any application requiring mechanical stability.
Understanding the Trade-offs
Selecting PTFE requires a clear understanding of its environmental limitations. Its strengths in one context can be significant weaknesses in another.
### The Primary Trade-off: Chemical vs. Radiation Resistance
The decision to use PTFE often hinges on this conflict. It is an unparalleled material for chemically harsh environments but a very poor choice for applications involving significant ionizing radiation.
### Inherent Mechanical Limitations
Even without radiation, PTFE is a relatively soft material. It is known to be prone to creep, or deformation over time when under a constant load.
### Manufacturing Complexity
PTFE cannot be processed using conventional melt-processing techniques like injection molding. This requires specialized manufacturing methods, which can impact design possibilities and cost.
Making the Right Choice for Your Application
Your final decision must be based on a clear-eyed assessment of the material's total operating environment.
- If your primary focus is chemical processing or laboratory use: PTFE is an elite choice, provided the environment is free from high-energy radiation.
- If your primary focus is high-frequency electrical insulation: PTFE's dielectric properties are superb, but you must ensure it will not be exposed to sterilization or other radiation sources.
- If your primary focus is an environment with ionizing radiation: Standard PTFE is unsuitable and will fail. You must seek out alternative, radiation-resistant polymers like PEEK or UHMWPE for these applications.
Ultimately, understanding a material's fundamental chemistry is the key to deploying it successfully.
Summary Table:
| Property | PTFE Performance | Notes |
|---|---|---|
| Chemical Resistance | Excellent | Resists most acids, bases, solvents |
| Radiation Resistance (Low-Energy) | Good | Resists UV, IR radiation |
| Radiation Resistance (High-Energy) | Poor | Degrades under gamma rays, electron beams |
| Thermal Stability | Up to 260°C (500°F) | Suitable for high-temperature environments |
| Electrical Insulation | Excellent | Ideal for high-frequency applications |
Need radiation-resistant or high-purity PTFE components for your application?
At KINTEK, we specialize in manufacturing precision PTFE seals, liners, and labware for the semiconductor, medical, and laboratory industries. Whether you require custom prototypes or high-volume production, our expertise ensures your components meet the exact demands of your operating environment—especially when radiation exposure is a concern.
Contact us today to discuss your specific requirements and explore how our tailored PTFE solutions can enhance your project's performance and reliability.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Bottles for Diverse Industrial Applications
- Customizable PTFE Seals Filter Holders for Versatile Applications
People Also Ask
- What factors should be considered when choosing between Nylon and PTFE? Select the Right Material for Your Application
- What industrial benefits do PTFE-machined parts offer? Achieve Peak Performance in Demanding Applications
- What are the main applications of PTFE type Teflon? Unlock Its Versatility for Your Industry
- What fabrication services are available for PTFE? Shearing, Stamping, Laser Cutting, Molding & Machining
- What are the unique properties of PTFE? Unlock Unmatched Performance in Demanding Applications



















