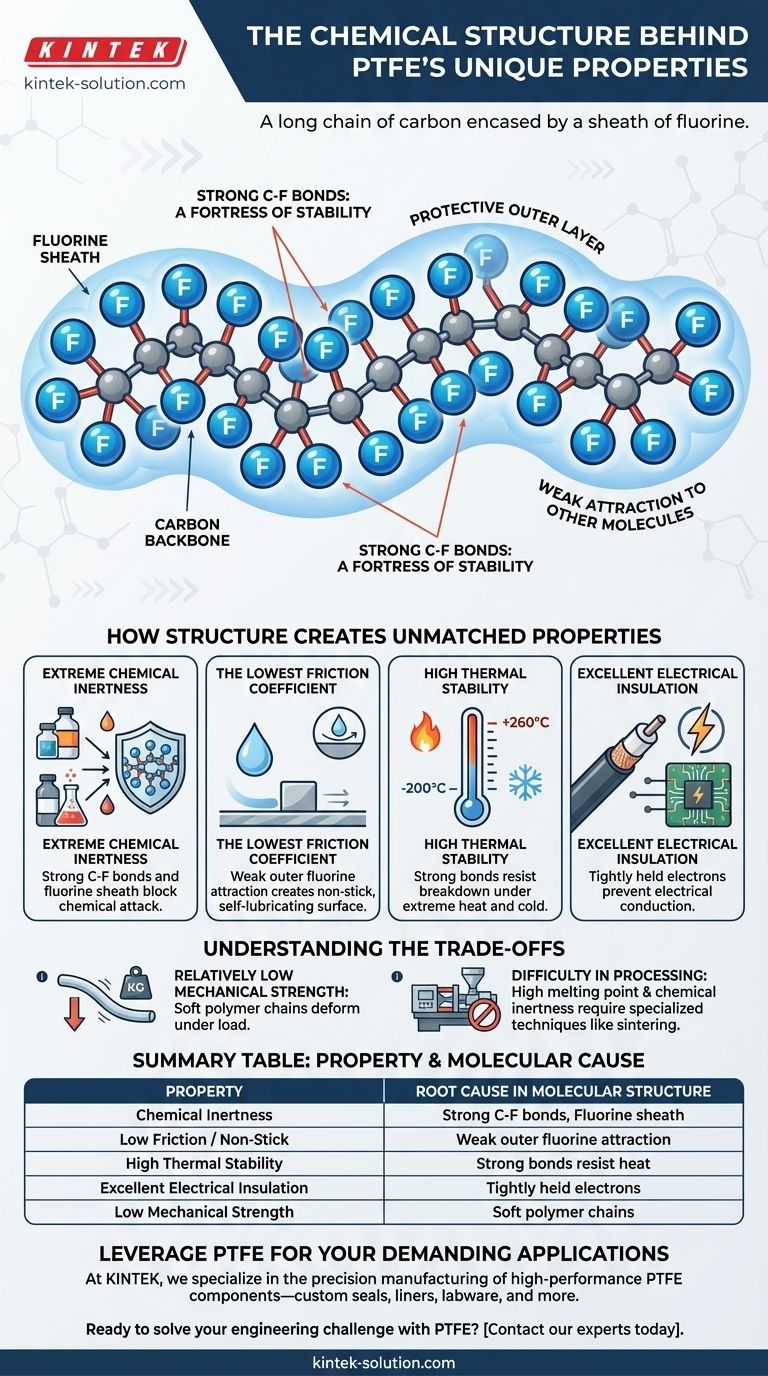
At its core, the unique properties of Polytetrafluoroethylene (PTFE) stem from its remarkably simple and stable chemical structure: a long chain of carbon atoms completely encased by a sheath of fluorine atoms. The immense strength of the carbon-fluorine bond provides incredible chemical and thermal stability, while the fluorine sheath itself creates an extremely low-energy, non-reactive surface, resulting in its famous non-stick and low-friction characteristics.
The source of PTFE's power is twofold: exceptionally strong chemical bonds prevent it from breaking down, while a protective outer layer of fluorine atoms repels almost everything it touches. This combination of structural integrity and surface-level indifference gives it properties unmatched by other polymers.

Deconstructing the PTFE Molecule
To understand why PTFE behaves so differently from other plastics, we must look at its atomic architecture. It is a polymer, a long chain of repeating molecular units, but the specific atoms involved make all the difference.
The Carbon Backbone
Like many common plastics (such as polyethylene), PTFE is built on a long, flexible backbone of carbon atoms linked together. This chain provides the fundamental structure of the material.
The Fluorine Sheath
The critical difference lies in what is attached to this carbon backbone. Where polyethylene has smaller hydrogen atoms, PTFE has larger fluorine atoms bonded to every available carbon site. These fluorine atoms are so densely packed that they form a continuous, protective "sheath" around the entire carbon chain.
The Carbon-Fluorine Bond: A Fortress of Stability
The bond between a carbon atom and a fluorine atom (C-F) is one of the strongest single bonds known in organic chemistry. It requires a tremendous amount of energy to break. This exceptional bond strength is the primary source of PTFE's resilience.
How Structure Creates Unmatched Properties
This unique molecular design directly translates into the macroscopic properties that make PTFE, often known by the brand name Teflon, so valuable across industries.
Extreme Chemical Inertness
The C-F bonds are incredibly stable and difficult for other chemicals to attack and break. Furthermore, the fluorine sheath acts as a physical barrier, preventing corrosive agents from even reaching the vulnerable carbon backbone. Only extreme conditions, like contact with molten alkali metals or hot fluorine gas, can degrade it.
The Lowest Friction Coefficient
The fluorine atoms in the outer sheath have very weak forces of attraction to other molecules. They are electrically stable and don't want to interact. When another material slides against a PTFE surface, there is virtually no molecular "stickiness," resulting in the lowest coefficient of friction of any known solid. This is the source of its non-stick quality.
High Thermal Stability
The immense strength of the C-F bond means the molecule doesn't vibrate apart or degrade easily when heated. This gives PTFE a very high melting point (327°C / 621°F) and a wide, stable operating temperature range from -200°C to +260°C (-328°F to +500°F).
Excellent Electrical Insulation
Fluorine atoms hold their electrons very tightly. This means there are no loose electrons to move through the material and conduct a current. This property, known as high dielectric strength, makes PTFE a superb insulator for high-frequency applications like coaxial cables and printed circuit boards.
Understanding the Trade-offs
No material is perfect, and PTFE's unique structure also creates limitations that are important to recognize.
Relatively Low Mechanical Strength
While chemically durable, PTFE is a relatively soft material. Compared to other engineering plastics, it has lower tensile strength and can be more susceptible to "creep" (slow deformation under constant load) and abrasion from sharp particles.
Difficulty in Processing
The same properties that make PTFE so resilient—chemical inertness and a high melting point—also make it difficult to process. It doesn't melt and flow like common plastics, meaning it cannot be easily injection molded or extruded. It often requires specialized techniques like sintering, a process of compacting and heating powder.
Making the Right Choice for Your Application
Understanding the link between PTFE's molecular structure and its properties allows you to apply it where it will provide the most value.
- If your primary focus is extreme chemical resistance: PTFE is an unparalleled choice for lining pipes, valves, and vessels in the chemical processing industry.
- If your primary focus is minimal friction: PTFE coatings on bearings, seals, and non-stick cookware provide a self-lubricating surface that is difficult to match.
- If your primary focus is high-frequency electrical insulation: PTFE's exceptional dielectric properties make it a top-tier material for high-performance cables and circuit boards.
- If your primary focus is high mechanical strength or wear resistance: You may need to consider a reinforced grade of PTFE or an alternative engineering plastic designed for high-stress applications.
By recognizing how its atomic structure governs its behavior, you can deploy PTFE with precision to solve your most demanding engineering challenges.
Summary Table:
| Property | Root Cause in Molecular Structure |
|---|---|
| Chemical Inertness | Extremely strong C-F bonds and a protective fluorine sheath. |
| Low Friction / Non-Stick | Outer fluorine atoms have weak attraction to other molecules. |
| High Thermal Stability | Strong C-F bonds resist breaking down under high heat. |
| Excellent Electrical Insulation | Fluorine atoms hold electrons tightly, preventing current flow. |
| Low Mechanical Strength | Relatively soft polymer chains can deform under load. |
Leverage the unique properties of PTFE for your most demanding applications.
At KINTEK, we specialize in the precision manufacturing of high-performance PTFE components—from custom seals and liners to complex labware. Whether you're in the semiconductor, medical, laboratory, or industrial sector, our expertise in custom fabrication ensures you get a component that perfectly balances PTFE's legendary chemical resistance and non-stick properties with the mechanical performance your application requires.
Ready to solve your engineering challenge with PTFE? Contact our experts today to discuss your project, from prototype to high-volume production.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Customizable PTFE Rods for Advanced Industrial Applications
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
People Also Ask
- What are the six common types of PTFE? Choose the Right Form for Your Application
- What are the fire safety properties of Teflon? Discover Its Non-Flammable Nature for Your Application
- What are the key properties of PTFE that make it valuable? Unlock Extreme Performance for Harsh Environments
- When did Teflon become a registered trademark, and what was an early use of the material? Discover Its Industrial Origins
- What are the molecular characteristics of PTFE? Unlocking the Secrets of Its Unmatched Performance
- What are the advantages of PTFE's impact resistance? Ensure Unmatched Durability in Harsh Environments
- Are there references available for PTFE chemical compatibility? Ensure Material Safety with the Right Guide
- What are the modified versions of PTFE and their properties? Enhance Performance for Demanding Applications



















