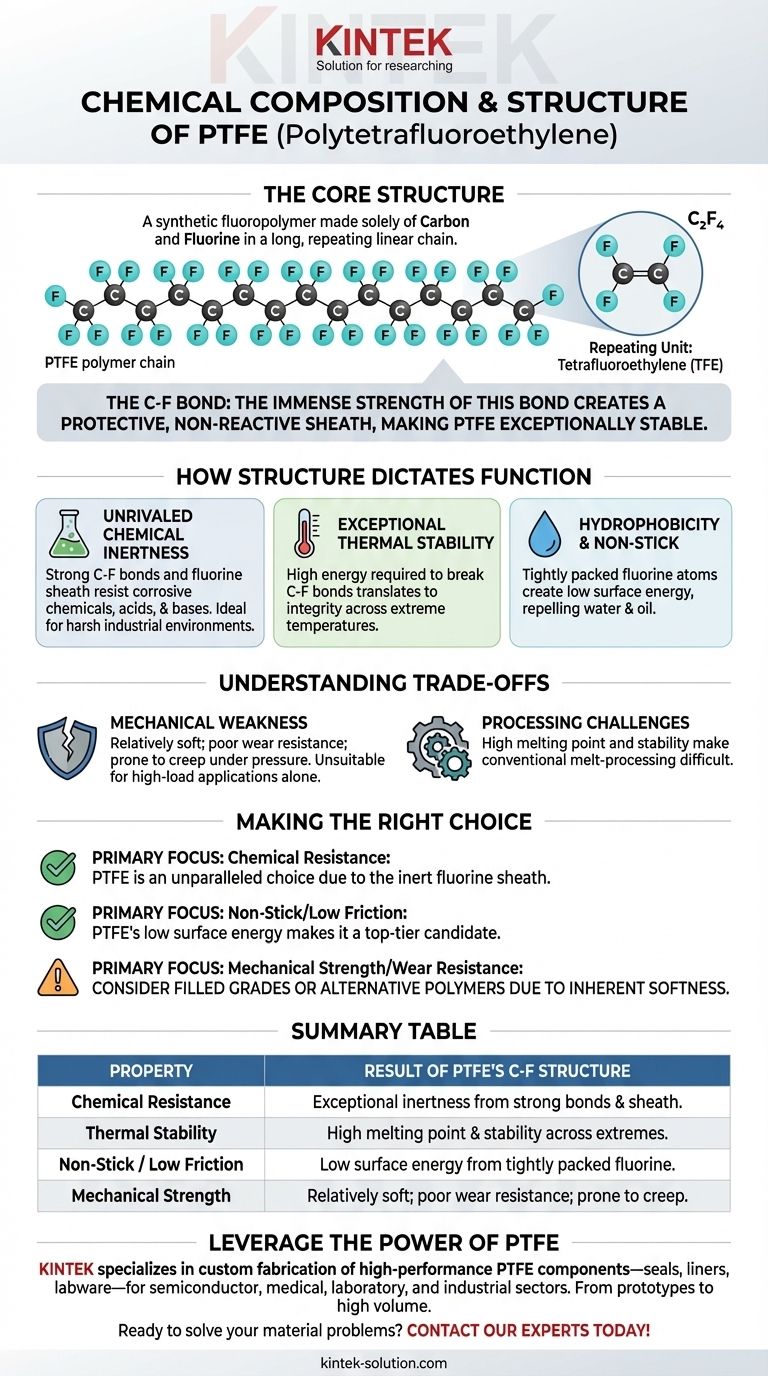
At its core, Polytetrafluoroethylene (PTFE) is a remarkably simple yet robust polymer. It is a synthetic fluoropolymer composed exclusively of two elements: carbon and fluorine. These atoms are arranged in a long, repeating linear chain, creating a high-molecular-weight compound that is the foundation for its well-known properties.
The immense strength of the carbon-fluorine bond is the single most important factor in understanding PTFE. This bond creates a protective, non-reactive sheath around a carbon backbone, rendering the entire molecule exceptionally stable and inert.

The Molecular Blueprint of PTFE
To understand why PTFE behaves the way it does, we must first examine its fundamental structure. It is a polymer, meaning it is a large molecule made up of many smaller, repeating units called monomers.
The Carbon Backbone
The skeleton of the PTFE molecule is a long, linear chain of carbon (C) atoms. This backbone provides the structural framework for the entire polymer.
The Fluorine Sheath
Each carbon atom in the backbone is bonded to two fluorine (F) atoms. Because fluorine atoms are relatively large and highly electronegative, they tightly pack around the carbon chain, creating a stable and protective "sheath."
This fluorine sheath effectively shields the more vulnerable carbon backbone from chemical attack. It is the primary reason for PTFE's extreme non-reactivity.
The Repeating Unit
The monomer, or the single repeating building block of PTFE, is tetrafluoroethylene (TFE). Its chemical formula is C₂F₄. Thousands of these TFE units link together in a process called polymerization to form the final PTFE molecule.
How Structure Dictates Function
The unique arrangement of carbon and fluorine atoms directly translates into the exceptional properties that define PTFE, widely known by the brand name Teflon.
Unrivaled Chemical Inertness
The carbon-fluorine bond is one of the strongest single bonds in organic chemistry. This strength, combined with the protective fluorine sheath, makes it incredibly difficult for other chemicals to break the molecule apart.
As a result, PTFE is resistant to almost all corrosive chemicals, acids, and bases, making it invaluable for use in harsh industrial environments.
Exceptional Thermal Stability
It takes a significant amount of energy to break the strong C-F bonds. This directly translates to high thermal stability, allowing PTFE to maintain its integrity across a wide range of extreme temperatures.
Hydrophobicity and Non-Stick Properties
The tightly packed fluorine atoms create a surface with very low energy. This low surface energy makes it difficult for other substances—including water and oil—to "wet" or adhere to it.
This is the principle behind PTFE's hydrophobic (water-repelling) and famous non-stick characteristics.
Understanding the Trade-offs
While its chemical structure provides incredible benefits, it also results in specific limitations that are critical to acknowledge for any technical application.
Mechanical Weakness
PTFE is a relatively soft material. It has poor resistance to wear and abrasion and can deform under pressure, a phenomenon known as "creep." This makes it unsuitable for high-load structural applications on its own.
Processing Challenges
The same chemical stability and high melting point that make PTFE so durable also make it difficult to process. It cannot be easily melt-processed using conventional techniques like injection molding that work for other thermoplastics.
Making the Right Choice for Your Goal
Understanding the direct link between PTFE's simple C-F structure and its properties is key to using it effectively.
- If your primary focus is chemical resistance: PTFE is an almost unparalleled choice due to the inert fluorine sheath protecting its carbon backbone.
- If your primary focus is creating a non-stick or low-friction surface: The low surface energy created by the tightly packed fluorine atoms makes PTFE a top-tier candidate.
- If your primary focus is mechanical strength or wear resistance: You must consider using filled grades of PTFE (mixed with materials like glass or carbon) or an entirely different polymer, as its inherent softness is a significant limitation.
Recognizing that PTFE's immense chemical strength and its mechanical weakness originate from the very same structure is the key to leveraging it correctly.
Summary Table:
| Property | Result of PTFE's C-F Structure |
|---|---|
| Chemical Resistance | Exceptional inertness due to strong C-F bonds and protective fluorine sheath. |
| Thermal Stability | High melting point and stability across extreme temperatures. |
| Non-Stick / Low Friction | Low surface energy from tightly packed fluorine atoms. |
| Mechanical Strength | Relatively soft with poor wear resistance; prone to creep. |
Leverage the Power of PTFE for Your Application
Understanding the link between PTFE's structure and its properties is the first step. The next is sourcing precision-manufactured components that meet your exact needs.
KINTEK specializes in the custom fabrication of high-performance PTFE components—including seals, liners, and labware—for the semiconductor, medical, laboratory, and industrial sectors. Whether you require prototypes or high-volume orders, our expertise ensures you get the chemical resistance and performance your application demands.
Ready to solve your most challenging material problems? Contact our experts today to discuss your project and receive a quote.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Bottles for Diverse Industrial Applications
People Also Ask
- What fabrication services are available for PTFE? Shearing, Stamping, Laser Cutting, Molding & Machining
- What are the unique properties of PTFE? Unlock Unmatched Performance in Demanding Applications
- What are the unique properties of PTFE? The 3 Pillars Driving Demand for High-Performance Parts
- What finishing techniques are effective for machined Teflon parts? Achieve Functional Performance and Dimensional Stability
- What industrial benefits do PTFE-machined parts offer? Achieve Peak Performance in Demanding Applications



















