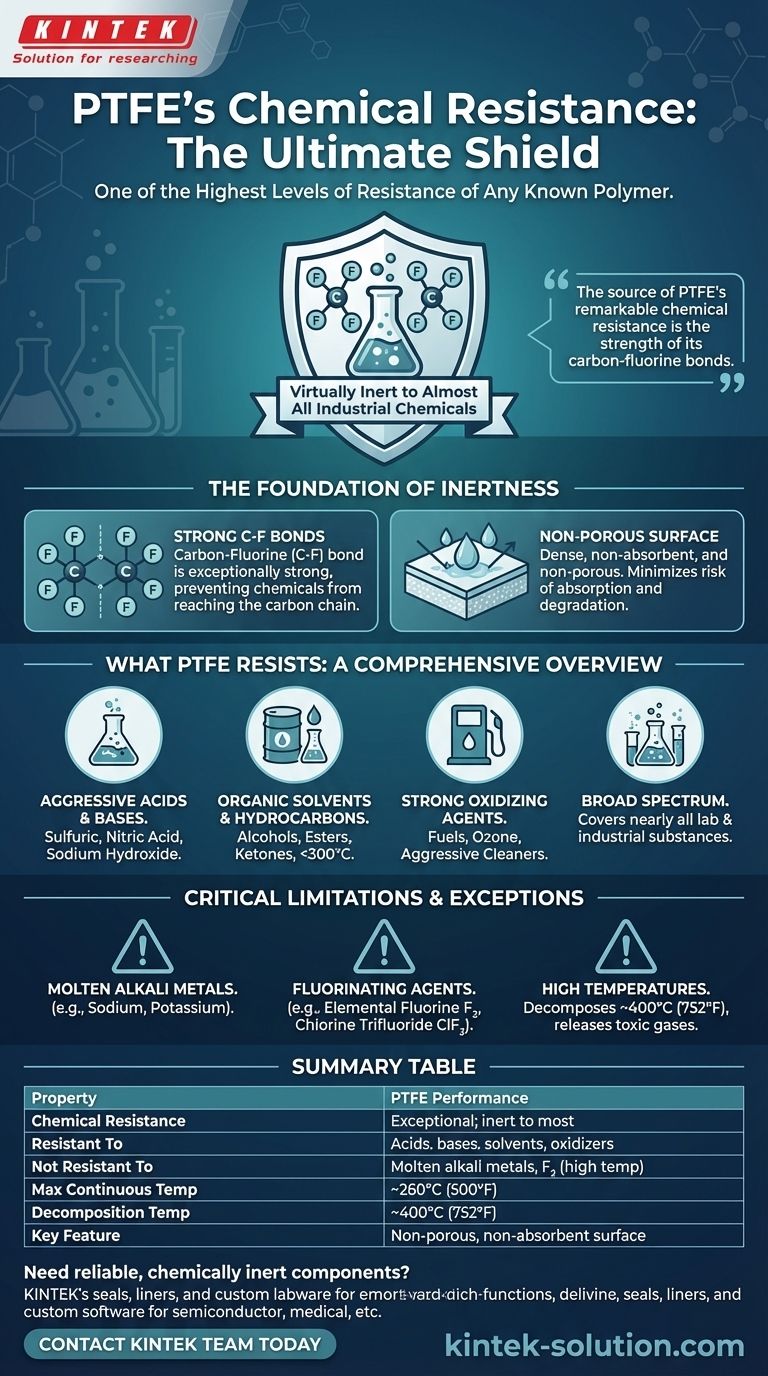
In short, Polytetrafluoroethylene (PTFE) has one of the highest levels of chemical resistance of any known polymer. It is virtually inert to almost all industrial chemicals, including concentrated acids, bases, solvents, and powerful oxidizing agents. The only common substances known to attack PTFE are molten alkali metals and highly reactive fluorinating agents like elemental fluorine at high temperatures.
The source of PTFE's remarkable chemical resistance is the strength of its carbon-fluorine bonds. This creates a stable, non-porous, and non-reactive material suitable for the most aggressive chemical environments, with only a few extreme exceptions.

The Foundation of PTFE's Inertness
To understand if PTFE is right for your application, you must first understand the source of its unique properties. Its resistance is not a surface-level feature but is inherent to its molecular structure.
The Strength of the Carbon-Fluorine Bond
PTFE is a fluoropolymer, meaning its molecular backbone consists of a chain of carbon atoms completely shielded by fluorine atoms.
The carbon-fluorine (C-F) bond is exceptionally strong and stable. This molecular shield effectively prevents chemicals from reaching and reacting with the vulnerable carbon chain, rendering the material almost entirely inert.
A Non-Porous, Non-Absorbent Surface
Beyond its molecular stability, PTFE's physical properties enhance its chemical performance. It is a dense material that does not absorb water and is non-porous.
This prevents chemicals from seeping into the material, minimizing the risk of absorption, degradation, or cross-contamination between processes.
What PTFE Resists: A Comprehensive Overview
PTFE's resistance is broad, covering nearly all substances you would encounter in laboratory or industrial settings.
Aggressive Acids and Bases
PTFE is impervious to both dilute and highly concentrated acids and bases. This includes substances like sulfuric acid, nitric acid, and sodium hydroxide, which would quickly degrade lesser materials.
Organic Solvents and Hydrocarbons
PTFE is insoluble in all known solvents below 300°C (572°F). It can be used confidently with alcohols, esters, ketones, and aliphatic, aromatic, or halogenated hydrocarbons without fear of dissolving or swelling.
Strong Oxidizing Agents
The material is highly resistant to powerful oxidizing agents, fuels, and ozone. This makes it ideal for applications involving aggressive cleaning agents or high-energy processes.
Understanding the Critical Limitations
While its resistance is exceptional, PTFE is not invincible. Understanding its specific vulnerabilities is critical for safety and application success.
The Few Chemical Exceptions
A small number of highly reactive substances can attack PTFE, typically under specific conditions of high temperature and pressure.
These exceptions are:
- Molten alkali metals (e.g., sodium, potassium)
- Elemental fluorine gas (F₂)
- Chlorine Trifluoride (ClF₃) and other potent fluorinating agents
For virtually all other applications, chemical compatibility is not a concern.
The Impact of Extreme Temperatures
The primary operational limit for PTFE is thermal, not chemical. It begins to decompose at temperatures around 400°C (752°F).
This thermal decomposition is a critical safety consideration, as it releases toxic fluorocarbon gases. Therefore, PTFE should never be used in applications where it could be exposed to such extreme temperatures.
Making the Right Choice for Your Application
Selecting PTFE should be a decision based on its unique profile of near-total chemical inertness balanced against its specific thermal and chemical limits.
- If your primary focus is handling a wide range of corrosive acids, solvents, or bases: PTFE is an industry-standard choice, offering unmatched reliability and purity.
- If your environment involves molten alkali metals or elemental fluorine: You must seek a specialized alternative, as PTFE will degrade in these specific conditions.
- If your application will experience temperatures approaching 400°C (752°F): You must consider the risk of thermal decomposition and select a material with a higher temperature rating.
Ultimately, for nearly any application demanding extreme chemical resistance at moderate temperatures, PTFE remains one of the most effective materials available.
Summary Table:
| Property | PTFE Performance |
|---|---|
| Chemical Resistance | Exceptional; inert to most chemicals |
| Resistant To | Concentrated acids, bases, solvents, oxidizing agents |
| Not Resistant To | Molten alkali metals, elemental fluorine (at high temp) |
| Max Continuous Temp | ~260°C (500°F) |
| Decomposition Temp | ~400°C (752°F) |
| Key Feature | Non-porous, non-absorbent surface |
Need reliable, chemically inert components for your demanding applications?
KINTEK specializes in manufacturing high-precision PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors. Our expertise ensures your equipment operates safely and efficiently in the most aggressive chemical environments.
Whether you require a custom prototype or a high-volume production run, we deliver solutions that meet your exact specifications. Contact our team today to discuss your project requirements and discover how KINTEK can support your success.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- What are some exceptional properties of PTFE? Unlock Unmatched Performance in Extreme Environments
- What are the primary applications of PTFE? Unlocking High-Performance Solutions
- Why are PTFE vials considered environmentally friendly? Reduce Lab Waste with Durable Reusables
- What are the common characteristics of Teflon? Unlocking Extreme Chemical and Thermal Resistance
- What industrial applications does PTFE have? Unlock Performance in Extreme Environments



















