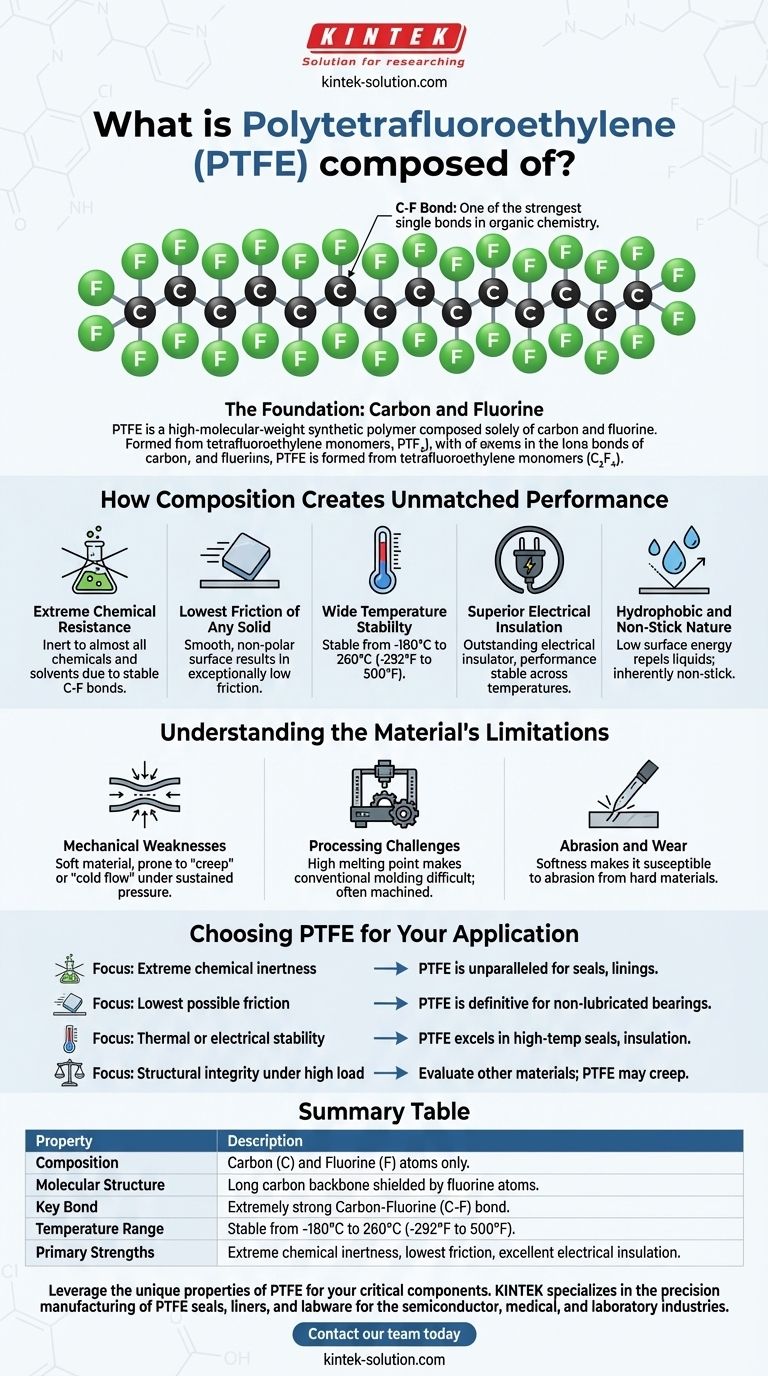
At its core, Polytetrafluoroethylene (PTFE) is a remarkably simple compound. It is a high-molecular-weight synthetic polymer, a fluoropolymer, consisting solely of two elements: carbon and fluorine. The material is created by joining long, repeating chains of the tetrafluoroethylene monomer (C₂F₄).
The extraordinary properties of PTFE—its extreme chemical inertness, non-stick nature, and low friction—are a direct result of its simple but powerful molecular structure: a stable carbon backbone completely shielded by a tight sheath of fluorine atoms.

The Foundation: Carbon and Fluorine
Understanding PTFE begins with its molecular architecture. The material's unique characteristics are not derived from a complex blend of chemicals but from the fundamental strength of a single type of chemical bond.
The Carbon Backbone
At the center of the PTFE molecule is a long, repeating chain of carbon atoms. This chain provides the structural framework for the polymer, much like the steel frame of a skyscraper.
The Fluorine Shield
Each carbon atom in the backbone is bonded to two fluorine atoms. These fluorine atoms are large and electronegative, effectively forming a tight, protective, and non-reactive "shield" around the entire carbon chain.
Why This Structure Is So Powerful
The bond between carbon and fluorine (C-F bond) is one of the strongest single bonds in organic chemistry. This immense strength and the complete fluorine coverage are the sources of almost all of PTFE's famous properties. The fluorine sheath creates a uniform, low-energy surface that repels nearly every other substance.
How Composition Creates Unmatched Performance
The simple combination of carbon and fluorine translates directly into a set of high-performance physical characteristics that are difficult to achieve with other materials.
Extreme Chemical Resistance
The powerful C-F bonds are incredibly stable and difficult for other chemicals to break. This makes PTFE inert to almost all chemicals and solvents, including strong acids and bases.
The Lowest Friction of Any Solid
The fluorine sheath creates an exceptionally smooth and non-polar molecular surface. Other molecules have little to nothing to "grab" onto, resulting in an extremely low coefficient of friction. This is why materials slide effortlessly over PTFE.
Wide Temperature Stability
A tremendous amount of thermal energy is required to disrupt the strong C-F bonds. Consequently, PTFE remains stable and functional across an exceptionally broad temperature range, typically from -180°C to 260°C (-292°F to 500°F).
Superior Electrical Insulation
The structure of PTFE does not allow for the easy movement of electrons. This makes it an outstanding electrical insulator whose performance is not significantly affected by changes in temperature or frequency.
Hydrophobic and Non-Stick Nature
The low surface energy created by the fluorine atoms means that liquids, including water and oils, cannot "wet" the surface. They bead up and roll off, making the material inherently hydrophobic and giving it its famous non-stick properties.
Understanding the Material's Limitations
Despite its remarkable strengths, PTFE is not the ideal choice for every application. Its unique composition also creates specific trade-offs that are critical to understand.
Mechanical Weaknesses
PTFE is a relatively soft material. Under sustained pressure, it is prone to "creep" or "cold flow," meaning it can slowly deform over time. While tough and flexible, its tensile strength is only average compared to other engineering plastics.
Processing Challenges
The same thermal stability that makes PTFE so useful also makes it difficult to process. Its high melting point and melt viscosity prevent it from being used in conventional methods like injection molding or extrusion. Instead, it is often machined from stock shapes, which can increase the cost of finished parts.
Abrasion and Wear
While its low friction minimizes surface wear in many sliding applications, the softness of the material can make it susceptible to abrasion from hard, rough counterparts or abrasive particles.
Choosing PTFE for Your Application
Your decision to use PTFE should be based on whether its unique strengths align with the primary demands of your project.
- If your primary focus is extreme chemical inertness: PTFE is an unparalleled choice for seals, linings, and components exposed to corrosive fluids.
- If your primary focus is the lowest possible friction: PTFE is the definitive material for non-lubricated bearings, slide plates, and low-friction coatings.
- If your primary focus is thermal or electrical stability: PTFE excels in high-temperature seals, cable insulation, and high-frequency electronic components where performance cannot vary.
- If your primary focus is structural integrity under high load: You should evaluate other materials, as PTFE's tendency to creep may lead to failure.
Ultimately, PTFE's simple two-element composition is the key to its powerful and highly specialized capabilities.
Summary Table:
| Property | Description |
|---|---|
| Composition | Carbon (C) and Fluorine (F) atoms only. |
| Molecular Structure | Long carbon chain backbone shielded by a sheath of fluorine atoms. |
| Key Bond | Extremely strong Carbon-Fluorine (C-F) bond. |
| Temperature Range | Stable from -180°C to 260°C (-292°F to 500°F). |
| Primary Strengths | Extreme chemical inertness, lowest friction, excellent electrical insulation. |
Leverage the unique properties of PTFE for your critical components. KINTEK specializes in the precision manufacturing of PTFE seals, liners, and labware for the semiconductor, medical, and laboratory industries. Whether you need a custom prototype or a high-volume order, our expertise ensures your parts meet the highest standards of performance and reliability. Contact our team today to discuss your specific application needs.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
People Also Ask
- What is the chemical formula and CAS number for PTFE? Unlocking Its Unique Properties
- What are the mechanical properties of FR4 PCB material? Understanding Rigidity, Thermal Limits & Cost
- What role does PTFE play in the electronics and electrical engineering sectors? The Ultimate Insulator and Protector
- What applications are PTFE laminated membrane filters suitable for? Master Filtration for Harsh Chemicals
- What are some alternatives to Teflon/PTFE? Explore High-Performance Polymers for Your Application
- What are the key properties of PTFE? A Guide to Its High-Performance Versatility
- How has PTFE production evolved since its discovery? From Lab Accident to High-Performance Polymer
- What chemicals is PTFE resistant to? Discover Its Near-Universal Chemical Inertness



















