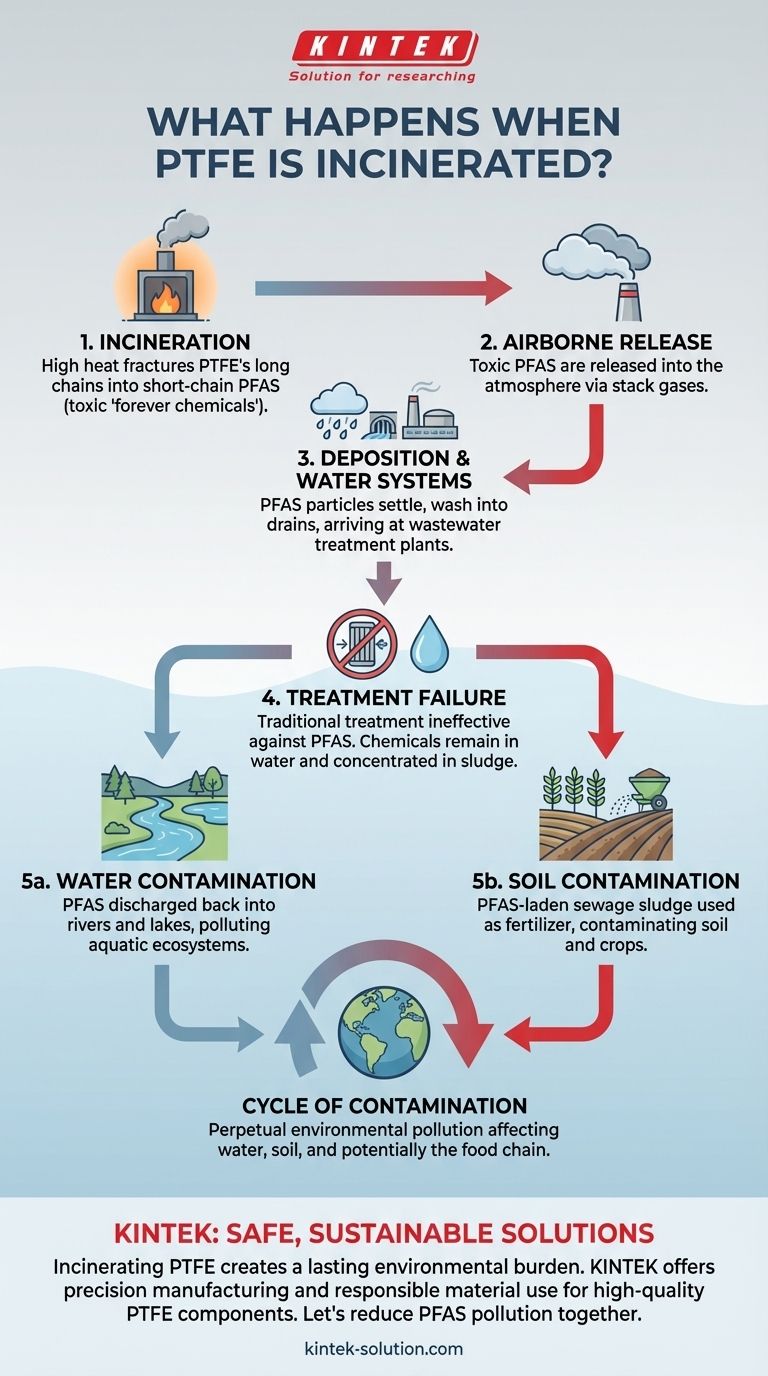
When PTFE is incinerated, it does not break down into harmless components. Instead, the high heat fractures its long molecular chains, creating and releasing shorter-chain PFAS (per- and polyfluoroalkyl substances) into the atmosphere. These toxic "forever chemicals" then enter the environment, posing a significant and persistent contamination risk.
The central problem with incinerating PTFE is that it transforms a solid, stable material into highly mobile and persistent chemical contaminants. This process doesn't destroy the hazard; it merely changes its form and spreads it into our water and soil.

The Contamination Pathway from Incineration
Incinerating materials containing PTFE, often known by the brand name Teflon, initiates a complex environmental chain reaction. The process converts a localized solid waste problem into a widespread chemical pollution issue.
From Long Chains to Short Chains
PTFE is a polymer, a very long molecule made of repeating carbon and fluorine atoms. This carbon-fluorine bond is one of the strongest in organic chemistry, which is why PTFE is so durable.
Incineration provides enough energy to break these long chains, but not enough to destroy the fundamental carbon-fluorine bonds. The result is a toxic mixture of smaller, more mobile PFAS compounds that are released with stack gases.
From Airborne Particles to Water Systems
These newly formed short-chain PFAS do not simply vanish. They travel through the air and are eventually deposited back onto the land and into water sources.
A primary route of contamination is through urban wastewater systems. The airborne particles settle and are washed into drains, ultimately arriving at wastewater treatment plants.
The Failure of Conventional Treatment
Wastewater treatment facilities are designed to remove biological waste and conventional pollutants, but they are completely ineffective against PFAS.
The chemical stability that makes PFAS persistent also means they pass through traditional filtration and treatment processes untouched. The chemicals remain in the treated water and become concentrated in the leftover sewage sludge.
The Consequence: A Cycle of Contamination
The failure to remove PFAS at the treatment stage creates a continuous cycle of environmental pollution. The problem is not contained; it is amplified.
Contaminated Water and Sludge
Because the PFAS are not removed, they are discharged directly back into rivers and lakes along with the treated wastewater.
Simultaneously, the sewage sludge, now highly concentrated with these forever chemicals, becomes a source of pollution itself.
Spreading the Problem to Soil
This contaminated sludge is often used as an agricultural fertilizer or sent to landfills. When applied to farmland, the PFAS are introduced directly into the soil.
From the soil, these chemicals can be absorbed by crops, leach into groundwater, or be carried into new waterways via agricultural runoff, perpetuating the cycle of contamination indefinitely.
How to Understand the Impact
Understanding the consequence of incinerating PTFE is crucial for evaluating waste management strategies and environmental policy.
- If your primary concern is waste disposal: Recognize that incineration is not a final solution for PTFE but rather a method of converting it into a more pervasive environmental contaminant.
- If your primary concern is water quality: The key takeaway is that PFAS released from incineration directly contaminate the water cycle in a way that our current infrastructure cannot mitigate.
- If your primary concern is public health: The creation of a contamination cycle that affects water, soil, and potentially the food chain is the most critical long-term risk.
Ultimately, incinerating PTFE transforms a single product into a lasting and widespread environmental burden.
Summary Table:
| Stage | Consequence |
|---|---|
| Incineration | High heat fractures PTFE's long chains into short-chain PFAS. |
| Airborne Release | Toxic PFAS are released into the atmosphere via stack gases. |
| Deposition | PFAS particles settle on land and wash into wastewater systems. |
| Water Contamination | PFAS pass through treatment plants, polluting rivers and lakes. |
| Soil Contamination | PFAS-laden sewage sludge is spread on farmland as fertilizer. |
Need a Safe, Sustainable Solution for Your PTFE Components?
Incinerating PTFE waste creates a lasting environmental burden. KINTEK offers an alternative through precision manufacturing and responsible material use.
We manufacture high-quality, durable PTFE components (seals, liners, labware, etc.) for the semiconductor, medical, laboratory, and industrial sectors. By prioritizing precision and offering custom fabrication from prototypes to high-volume orders, we help our partners maximize product lifespan and minimize waste.
Let's work together to reduce PFAS pollution. Discuss your application and explore how our components can meet your needs reliably and responsibly.
Contact KINTEK today to learn more.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
People Also Ask
- How does PTFE contribute to low friction and wear resistance? Achieve Superior Performance with Advanced Materials
- Why is CNC machining preferred for Teflon parts over other methods? Unlock Precision & Complex Designs
- What are the future considerations for machining Teflon? Mastering Material Challenges with Smart Tech
- What tips can improve Teflon machining results? Master Sharp Tools, Heat Control, and Rigid Support
- What are the key advantages of PTFE? Unmatched Performance for Extreme Environments



















