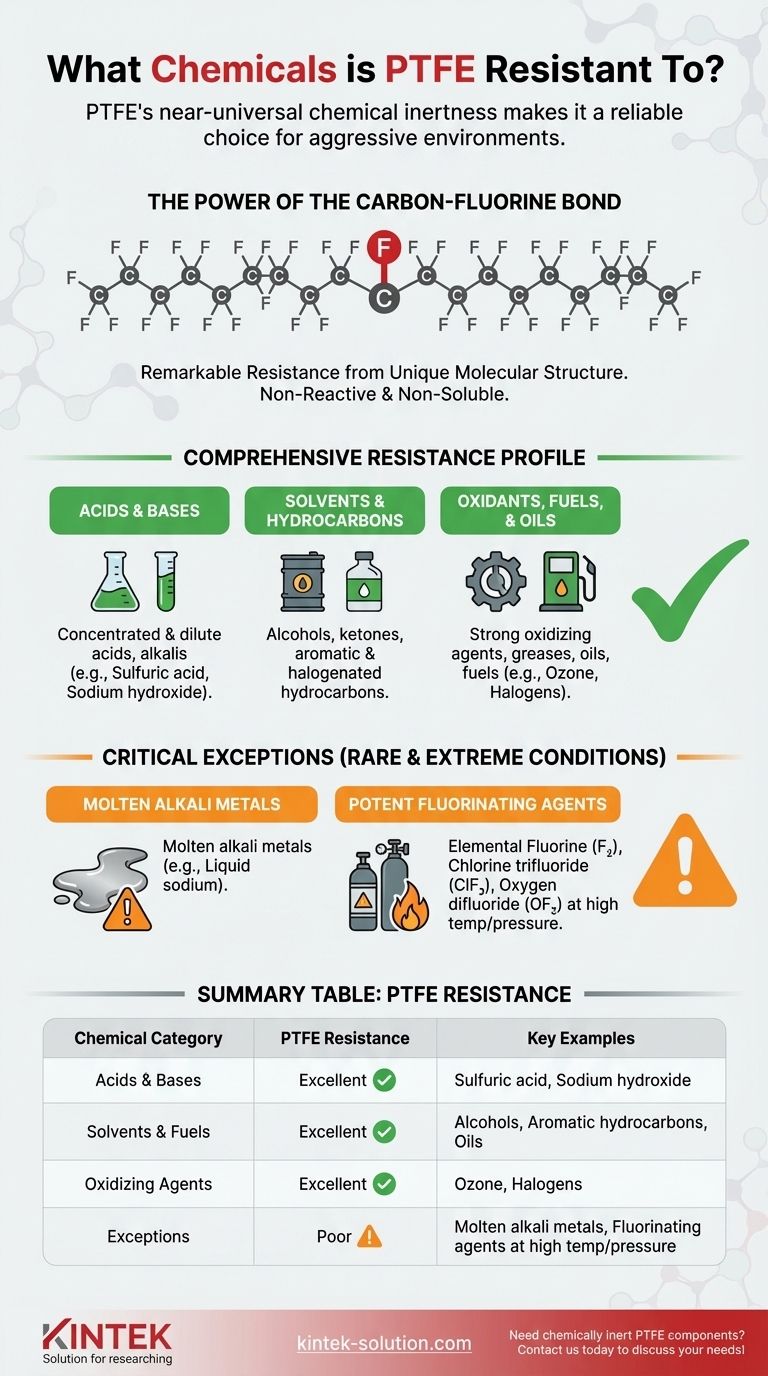
To put it directly, Polytetrafluoroethylene (PTFE) is resistant to nearly every chemical it encounters. Its chemical inertness is one of its most defining properties, making it stable when exposed to a vast range of substances including concentrated acids, bases, alcohols, solvents, fuels, oils, and strong oxidants.
The core principle to understand is that PTFE's chemical resistance is almost total. The exceptions are so rare and extreme—primarily involving molten alkali metals and potent fluorinating agents at high temperatures—that for the vast majority of industrial and laboratory applications, PTFE is considered virtually inert.

The Foundation of PTFE's Inertness
The remarkable chemical resistance of PTFE isn't arbitrary; it stems directly from its unique molecular structure. This structure is the key to why it performs so well in aggressive environments.
The Power of the Carbon-Fluorine Bond
At its core, PTFE consists of a long chain of carbon atoms completely shielded by a sheath of fluorine atoms. The carbon-fluorine (C-F) bond is one of the strongest single bonds in organic chemistry.
This powerful bond is incredibly stable and difficult to break, preventing other chemicals from reacting with the carbon backbone of the polymer.
A Non-Reactive and Non-Soluble Material
Because of this molecular stability, PTFE does not react with common substances like oxygen or water.
Furthermore, it is not soluble in any known solvent at room temperature. This prevents chemical attack through dissolution, a common failure mode for other plastics.
Comprehensive Resistance Profile
PTFE’s stability translates to exceptional performance across nearly all classes of chemicals, making it a default choice for demanding applications.
Acids and Bases
PTFE is highly resistant to both concentrated and dilute acids and alkalis. This includes aggressive chemicals like sulfuric acid, hydrochloric acid, and sodium hydroxide.
Solvents and Hydrocarbons
It shows no degradation when exposed to a wide array of organic compounds, including alcohols, ketones, aromatic hydrocarbons, and halogenated hydrocarbons.
Oxidants, Fuels, and Oils
The material remains stable against strong oxidizing agents, such as ozone and halogens, as well as common greases, oils, and fuels.
Understanding the Critical Exceptions
While its resistance is broad, it is not absolute. For safety-critical applications, it is essential to know the specific, and rare, conditions under which PTFE can be attacked.
Molten Alkali Metals
The most cited exception is molten alkali metals, such as liquid sodium. These highly reactive metals are powerful enough to disrupt the C-F bond.
Potent Fluorinating Agents
Certain highly aggressive chemicals can attack PTFE, typically under conditions of elevated temperature and pressure.
These include elemental **fluorine (F₂) gas, chlorine trifluoride (ClF₃), and oxygen difluoride (OF₂) **. These are some of the most powerful fluorinating agents known and are rarely encountered outside of specialized industrial processes.
The Role of Extreme Conditions
It is crucial to emphasize that these exceptions are relevant primarily in extreme environments. At room temperature and standard pressure, PTFE's chemical resistance remains virtually total.
Making the Right Choice for Your Application
Your decision to use PTFE should be based on a clear understanding of your operating environment.
- If your primary focus is general chemical processing: PTFE is an exceptionally safe and reliable choice for handling the vast majority of acids, bases, solvents, and industrial chemicals.
- If your primary focus is a highly specialized, extreme environment: You must verify that your process does not involve molten alkali metals or high-temperature, high-pressure fluorinating agents.
Ultimately, PTFE's near-universal chemical inertness makes it one of the most reliable materials available for chemically aggressive applications.
Summary Table:
| Chemical Category | PTFE Resistance | Key Examples |
|---|---|---|
| Acids & Bases | Excellent | Sulfuric acid, Hydrochloric acid, Sodium hydroxide |
| Solvents & Fuels | Excellent | Alcohols, Ketones, Aromatic hydrocarbons, Oils |
| Oxidizing Agents | Excellent | Ozone, Halogens |
| Exceptions | Poor | Molten alkali metals, Fluorinating agents at high temp/pressure |
Need chemically inert PTFE components for your application? KINTEK specializes in manufacturing high-precision PTFE seals, liners, and labware for semiconductor, medical, laboratory, and industrial sectors. Whether you require custom prototypes or high-volume production, our expertise ensures reliable performance in aggressive chemical environments. Contact us today to discuss your specific needs!
Visual Guide
Related Products
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
People Also Ask
- What is PTFE commonly known as and when was it developed? The 'Plastics King' for Extreme Performance
- How does PTFE perform under high temperatures? Leverage Its Exceptional Thermal Stability Up to 260°C
- How does PTFE perform at low temperatures? Unlock Reliable Cryogenic Performance
- What is the role of persulphate in PTFE production? Unlocking the Key to Polymerization
- What are the thermal and electrical properties of PTFE? A Guide to Its Extreme Performance
- How is PTFE granular resin produced? The Key to Creating Robust, Machinable Components
- Are there regulations or restrictions on PTFE and other PFAS? Navigating the Global Shift Away from PFAS
- What are the key properties of PTFE (Polytetrafluoroethylene)? Unlock Superior Performance in Harsh Environments



















