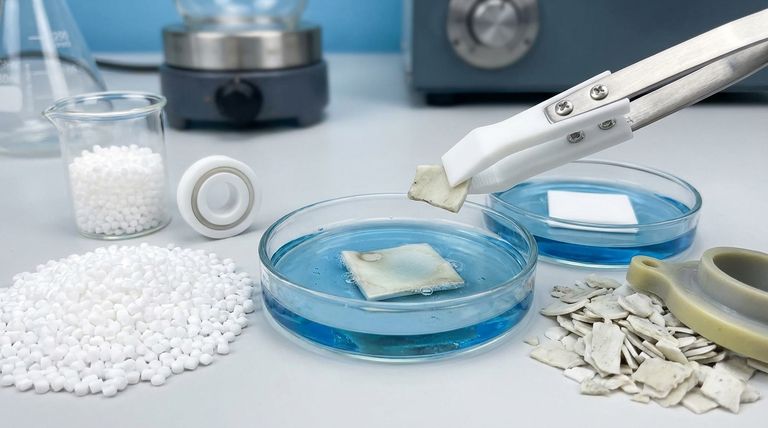
Color change in reprocessed Polytetrafluoroethylene (PTFE) is a direct indicator of its history and composition. The primary causes are the presence of foreign additives from its original use, reactions involving micro-impurities during the reprocessing cycle, and fundamental alterations to the PTFE molecular structure itself.
While often viewed as a cosmetic issue, a change in color in reprocessed PTFE should be treated as a critical signal. It can indicate a potential reduction in the material's most valued property: its exceptional chemical inertness.
The Three Primary Drivers of Color Change
Understanding why reprocessed PTFE changes color requires looking at what happens both on the surface and at the molecular level. These factors can occur individually or in combination.
Contamination from Foreign Additives
Many virgin PTFE products contain fillers to enhance specific properties like wear resistance or thermal conductivity.
A common additive is titanium dioxide (TiO2), which is used as a white pigment. During reprocessing, it can be difficult to completely remove these additives, leading to off-white, grey, or other discolorations in the final material.
Reactions from Micro-Impurities
The reprocessing cycle itself can introduce new variables that cause color shifts.
Micro-impurities trapped within the material can react with processing aids, such as coolants or lubricants, used during the grinding or heating stages. These chemical reactions can create new colored compounds within the PTFE matrix.
Alterations to the Molecular Structure
This is the most significant cause of color change because it directly impacts performance.
PTFE's incredible stability comes from a strong backbone of carbon atoms completely shielded by fluorine atoms. The high temperatures and mechanical stresses of reprocessing can dislodge fluorine atoms, exposing the underlying carbon atoms. This molecular-level damage is a primary source of discoloration.
Understanding the Trade-offs: The Impact on Performance
A shift in color is more than an aesthetic flaw; it is a warning sign that the material's fundamental properties may have been compromised.
Reduced Chemical Resistance
When fluorine atoms are removed, the exposed carbon chain becomes a weak point. This reduces the material's chemical inertness, making it more susceptible to attack by aggressive chemicals it could previously withstand. This degradation is the single greatest risk associated with using reprocessed PTFE in demanding environments.
The Question of Consistency
Unlike virgin PTFE, which has a tightly controlled manufacturing process, reprocessed PTFE comes from varied sources with different histories. This inherent variability means that color, purity, and performance can differ significantly from batch to batch, introducing unpredictability into your application.
Making the Right Choice for Your Application
Evaluating reprocessed PTFE requires aligning its known variables with the specific demands of your project. The color is your first clue to its suitability.
- If your primary focus is cost savings for non-critical components: Reprocessed PTFE may be a viable option, but rigorous batch testing is essential to ensure it meets minimum performance standards.
- If your primary focus is high purity or maximum chemical resistance: Virgin PTFE is the only choice that guarantees the molecular integrity and inertness required for medical, semiconductor, or aggressive chemical applications.
- If your primary focus is visual consistency: You must assume that color variation is a standard characteristic of reprocessed PTFE and design accordingly.
Ultimately, understanding the root cause of color change empowers you to make an informed decision based on performance risk rather than appearance alone.
Summary Table:
| Cause of Color Change | Impact on PTFE | Key Takeaway |
|---|---|---|
| Foreign Additives (e.g., TiO₂) | Contamination from original fillers | Indicates incomplete purification |
| Reactions with Micro-Impurities | Formation of new colored compounds | Signals potential chemical instability |
| Molecular Structure Damage | Loss of fluorine atoms, exposed carbon chain | Critical Warning: Significantly reduces chemical inertness |
Don't let color changes compromise your project's integrity. For applications where chemical purity, material consistency, and performance are non-negotiable—such as in semiconductor, medical, or laboratory settings—virgin PTFE is the only reliable choice.
At KINTEK, we specialize in manufacturing high-purity, custom PTFE components (seals, liners, labware, and more) from prototypes to high-volume orders. Our commitment to precision production ensures your materials deliver the exceptional chemical resistance and reliability you require.
Contact our experts today to discuss your specific needs and ensure your application is built on a foundation of guaranteed performance.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Customizable PTFE Rods for Advanced Industrial Applications
People Also Ask
- How is ePTFE structured and what are its properties? Unlock Advanced Performance with Microporous PTFE
- What are the six common types of PTFE? Choose the Right Form for Your Application
- What quality control measures are used in PTFE production? Ensure Material Integrity for Your Application
- Why is testing PTFE materials important for electrical applications? Ensure Performance & Safety
- What mechanical properties make PTFE suitable for industrial applications? Leverage Low Friction & Chemical Resistance
- What is the role of persulphate in PTFE production? Unlocking the Key to Polymerization
- What is the chemical composition of PTFE? Unlocking the Power of Carbon-Fluorine Bonds
- What is PTFE and why is it significant? Unlock the Power of a High-Performance Polymer



















