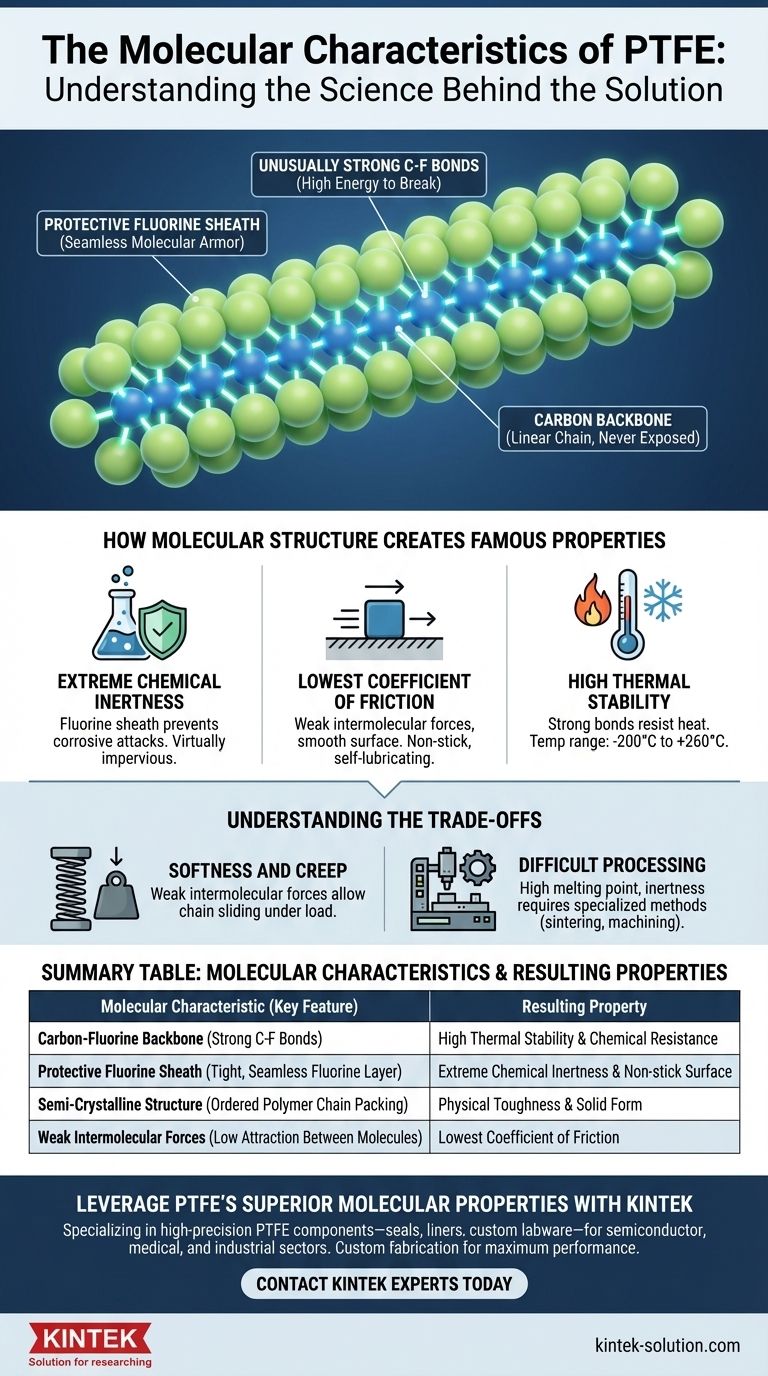
At its most fundamental level, Polytetrafluoroethylene (PTFE) is a simple yet remarkably robust linear polymer. Its defining molecular characteristics are a long chain of carbon atoms completely surrounded and protected by fluorine atoms. This structure results in exceptionally strong carbon-fluorine bonds and a semi-crystalline arrangement, which are directly responsible for its famous properties.
The extraordinary properties of PTFE—its extreme chemical inertness and the lowest coefficient of friction of any solid—are a direct result of its molecular architecture. Strong, stable carbon-fluorine bonds create a seamless, non-reactive "shield" around the polymer's carbon backbone.
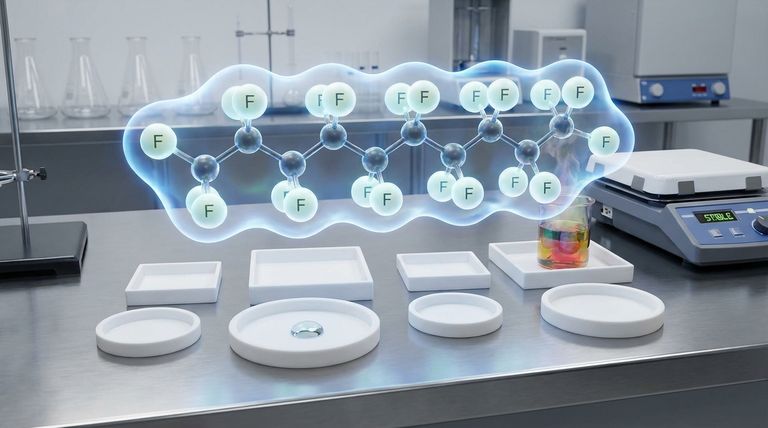
The Architecture of a PTFE Molecule
To understand why PTFE behaves the way it does, we must first examine its building blocks. Its properties are not random; they are a direct consequence of its chemical makeup.
The Carbon-Fluorine Backbone
At the center of a PTFE molecule is a long, repeating chain of carbon atoms. This forms the "backbone" of the polymer.
However, unlike in many other polymers, this carbon backbone is never exposed. Each carbon atom is bonded to two fluorine atoms.
The Protective Fluorine Sheath
The fluorine atoms are significantly larger than the carbon atoms they are bonded to. Because of this, they effectively wrap around the carbon backbone, creating a tight, uniform, and seamless protective sheath.
This "fluorine sheath" is the most critical feature of the PTFE molecule. It acts like molecular armor, preventing almost anything from reaching and reacting with the vulnerable carbon chain inside.
Unusually Strong Bonds
The bond between carbon and fluorine (C-F) is one of the strongest single bonds in organic chemistry.
It requires a tremendous amount of energy—either thermal or chemical—to break this bond. This inherent stability is the source of PTFE's high-temperature performance and chemical resistance.
How Molecular Structure Creates Famous Properties
The unique architecture of the PTFE molecule directly translates into the macroscopic properties that make the material so valuable in industrial, commercial, and medical applications.
Extreme Chemical Inertness
Because the carbon backbone is completely shielded by a tightly packed layer of chemically stable fluorine atoms, corrosive chemicals simply cannot find a point of attack. This molecular shield is why PTFE is virtually impervious to chemical attack.
The Lowest Coefficient of Friction
The fluorine sheath creates an extremely smooth, low-energy surface at the molecular level. The fluorine atoms have very weak intermolecular forces, meaning they do not attract or "stick" to other molecules.
This causes other materials to slide off effortlessly, giving PTFE its signature non-stick quality and the lowest coefficient of friction of any known solid material.
High Thermal Stability
The immense strength of the carbon-fluorine bonds means the molecule resists being broken apart by heat. This allows PTFE to maintain its integrity and performance across a vast temperature range, typically from –200°C to +260°C.
Crystallinity and Form
As a long, linear polymer, PTFE chains can pack together in an orderly, crystalline fashion in certain regions.
This semi-crystalline structure (typically 50-70% crystallinity) contributes to its physical toughness and form as a solid material, distinguishing it from lower-molecular-weight fluorocarbons that are oils or waxes.
Understanding the Trade-offs
No material is perfect, and the very molecular traits that give PTFE its strengths also lead to certain limitations.
Softness and Creep
The same weak intermolecular forces that create low friction also mean that the polymer chains can slide past one another when placed under a sustained load. This can lead to a slow deformation known as "creep."
Difficult Processing
PTFE’s high melting point (~327°C) and chemical inertness make it impossible to process using conventional, cost-effective melt-processing techniques common for other plastics. It must be formed using more specialized (and often more expensive) methods like sintering or machining.
Making the Right Choice for Your Application
Understanding the link between PTFE's molecular structure and its performance characteristics is key to using it effectively.
- If your primary focus is chemical resistance: PTFE's fluorine-shielded backbone makes it the definitive choice for handling highly corrosive materials.
- If your primary focus is low friction: Its smooth, low-energy molecular surface provides unparalleled non-stick and self-lubricating performance for bearings, seals, and coatings.
- If your primary focus is thermal stability: The immense strength of its carbon-fluorine bonds ensures it maintains structural integrity in environments where nearly all other polymers would fail.
By understanding PTFE at the molecular level, you can leverage its unique strengths with confidence and precision.
Summary Table:
| Molecular Characteristic | Key Feature | Resulting Property |
|---|---|---|
| Carbon-Fluorine Backbone | Strong C-F bonds | High thermal stability & chemical resistance |
| Protective Fluorine Sheath | Tight, seamless fluorine layer | Extreme chemical inertness & non-stick surface |
| Semi-Crystalline Structure | Ordered polymer chain packing | Physical toughness & solid form |
| Weak Intermolecular Forces | Low attraction between molecules | Lowest coefficient of friction |
Leverage PTFE's Superior Molecular Properties with KINTEK
Understanding PTFE's molecular architecture is the first step. Applying it effectively is the next. At KINTEK, we specialize in manufacturing high-precision PTFE components—including seals, liners, and custom labware—that harness these unique characteristics for demanding applications in the semiconductor, medical, laboratory, and industrial sectors.
Our expertise ensures that every component delivers the maximum chemical resistance, thermal stability, and non-stick performance that PTFE is famous for. Whether you need prototypes or high-volume orders, we provide custom fabrication tailored to your exact requirements.
Ready to integrate PTFE's unmatched properties into your application? Contact our experts today to discuss your project and discover how KINTEK's precision PTFE solutions can solve your most challenging problems.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Customizable PTFE Rods for Advanced Industrial Applications
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
People Also Ask
- What are the three ingredients used to synthesize TFE, and under what conditions are they combined? Master the High-Temp Pyrolysis Process
- How is PTFE used in the electrical industry? Unlock Superior Insulation for High-Frequency Applications
- Why is PTFE considered non-reactive? The Power of an Unbreakable Molecular Bond
- What chemicals is Teflon resistant to? The Ultimate Guide to PTFE Chemical Inertness
- What is virgin PTFE and what are its typical applications? Unlock the Power of Pure Performance
- What are the safety considerations when using Teflon-coated cookware? Ensure Safe Cooking with Proper Heat Management
- What is suspension polymerization and what does it produce? A Guide to Granular Polymers for Molding
- What temperature range can Teflon withstand? From Cryogenic -328°F to High Heat 500°F



















