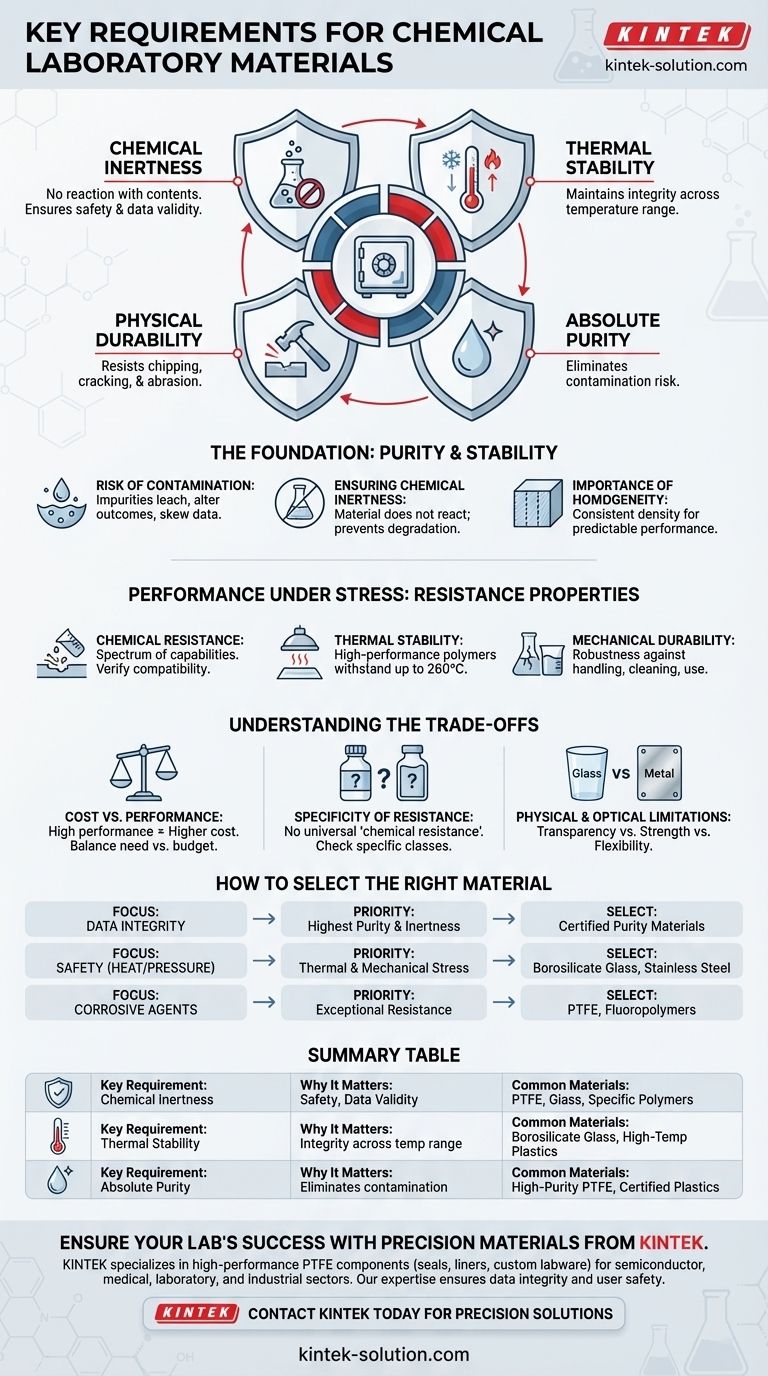
The essential requirements for materials used in chemical laboratories hinge on four critical pillars: chemical inertness, thermal stability, physical durability, and absolute purity. These materials must be fundamentally safe, stable, and completely non-reactive with the substances they contain to ensure both user safety and the integrity of experimental data.
The core challenge in laboratory material selection is not just finding a material that resists a single harsh condition, but choosing one that maintains its integrity across a range of chemical, thermal, and physical stresses, thereby guaranteeing both safety and the validity of scientific results.

The Foundation: Purity and Stability
The reliability of any chemical experiment begins with the purity and stability of the equipment used. These are not passive qualities but active guards against invalid results.
The Risk of Contamination
Any impurity in a lab material, from beakers to tubing, can potentially leach into a chemical solution. This contamination can alter reaction outcomes, skew analytical measurements, and render experimental data useless.
High-purity materials ensure that the only substances present in your experiment are the ones you intentionally put there.
Ensuring Chemical Inertness
A material is considered stable or inert when it does not chemically react with the substances it holds. This prevents the material itself from degrading and, more importantly, from becoming an unintended reactant in your experiment.
This property is fundamental to safety, preventing container failure when exposed to corrosive acids, bases, or solvents.
The Importance of Homogeneity
A material with homogeneous density provides consistent and predictable performance. It ensures that there are no hidden weak spots that could fail under thermal or physical stress, and that its resistance properties are uniform across its entire surface.
Performance Under Stress: Resistance Properties
A laboratory environment subjects materials to a wide range of extreme conditions. A material's ability to withstand these forces without failing is paramount.
Chemical Resistance: The First Line of Defense
Chemical resistance is the ability to withstand degradation from corrosive substances. This is not a single property but a spectrum of capabilities.
A material resistant to strong acids might be vulnerable to organic solvents, and vice-versa. Understanding the specific chemicals involved is crucial for proper material selection.
Thermal Stability: Handling the Heat
Experiments can involve temperatures ranging from cryogenic lows to hundreds of degrees Celsius. Materials must maintain their structural integrity and inertness across their specified working temperature range.
For example, high-performance polymers are often chosen for their ability to withstand working temperatures up to 260 °C (500 °F) without melting, warping, or degrading.
Mechanical Durability: Surviving the Environment
Beyond chemical and thermal stress, lab materials must be physically robust. They need to resist chipping, cracking, and abrasion from regular handling, cleaning, and use.
The choice between a brittle material like glass and a more ductile one like a polymer often comes down to the required balance between thermal/chemical resistance and mechanical durability.
Understanding the Trade-offs
No single material is perfect for every laboratory application. Selection always involves balancing competing properties and accepting certain compromises.
The Cost vs. Performance Balance
The highest-performing materials often come with the highest price tag. While a specialized fluoropolymer might offer superior resistance, its cost may be prohibitive for general-purpose use where borosilicate glass or polypropylene would suffice.
Specificity of Resistance
It is a common pitfall to assume "chemical resistance" is a universal trait. A material that is ideal for inorganic chemistry may perform poorly with organic solvents. Always verify a material's compatibility with the specific class of chemicals you are using.
Physical and Optical Limitations
Material choice also impacts usability. Glass is transparent, which is critical for observing reactions, but it is fragile. Metals are strong but opaque and can be reactive. Plastics can be flexible and durable but may have lower thermal limits and can sometimes absorb trace chemicals.
How to Select the Right Material for Your Application
Your final decision should be guided by the most critical demand of your specific task.
- If your primary focus is data integrity in trace analysis: Prioritize materials with the highest certified purity and chemical inertness to prevent sample contamination.
- If your primary focus is safety in high-temperature or high-pressure reactions: Select materials specifically rated for the thermal and mechanical stresses of your experiment, such as borosilicate glass or stainless steel.
- If your primary focus is handling highly corrosive agents: Choose a material, such as PTFE or other fluoropolymers, known for its exceptional resistance to your specific class of chemicals.
Ultimately, choosing the right material is the first and most critical step in ensuring safe, repeatable, and accurate scientific work.
Summary Table:
| Key Requirement | Why It Matters | Common Materials |
|---|---|---|
| Chemical Inertness | Prevents reaction with contents, ensuring safety and data validity. | PTFE, Glass, Specific Polymers |
| Thermal Stability | Maintains integrity across a wide temperature range (e.g., up to 260°C). | Borosilicate Glass, High-Temp Plastics |
| Physical Durability | Resists chipping, cracking, and abrasion from daily use. | Stainless Steel, Robust Polymers |
| Absolute Purity | Eliminates risk of contamination that can skew analytical results. | High-Purity PTFE, Certified Plastics |
Ensure Your Lab's Success with Precision Materials from KINTEK
Selecting the right material is the foundational step for safe, reliable, and accurate scientific work. KINTEK specializes in manufacturing high-performance PTFE components—including seals, liners, and custom labware—that meet the strictest requirements for chemical inertness, thermal stability, and purity.
Whether you are working in the semiconductor, medical, laboratory, or industrial sector, our expertise in custom fabrication—from prototypes to high-volume orders—ensures you get components that guarantee data integrity and user safety.
Let us help you mitigate risk and enhance your experimental outcomes. Contact KINTEK today to discuss your specific material needs and receive a quote for precision-engineered solutions.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
People Also Ask
- What are the benefits of PTFE shovels being antistatic? Prevent Fires & Protect Sensitive Electronics
- What temperature resistance do PTFE filters offer? Unmatched Thermal Stability from -200°C to +260°C
- What role do sealing properties play in the effectiveness of PTFE/silicone septums? Ensure Sample Integrity and Data Accuracy
- What temperature range can PTFE vials withstand? From -200°C to +260°C for Extreme Applications
- Why are PTFE shovels considered cost-effective? Maximize ROI with Superior Durability
- What temperature range can PTFE Tri-Clamp gaskets withstand? -200°C to 260°C for Extreme Applications
- What are the key applications of the PTFE bottle? Ensure Chemical Safety and Sample Purity
- What makes PTFE lined vials easy to clean? The Science Behind Their Non-Stick, Inert Surface