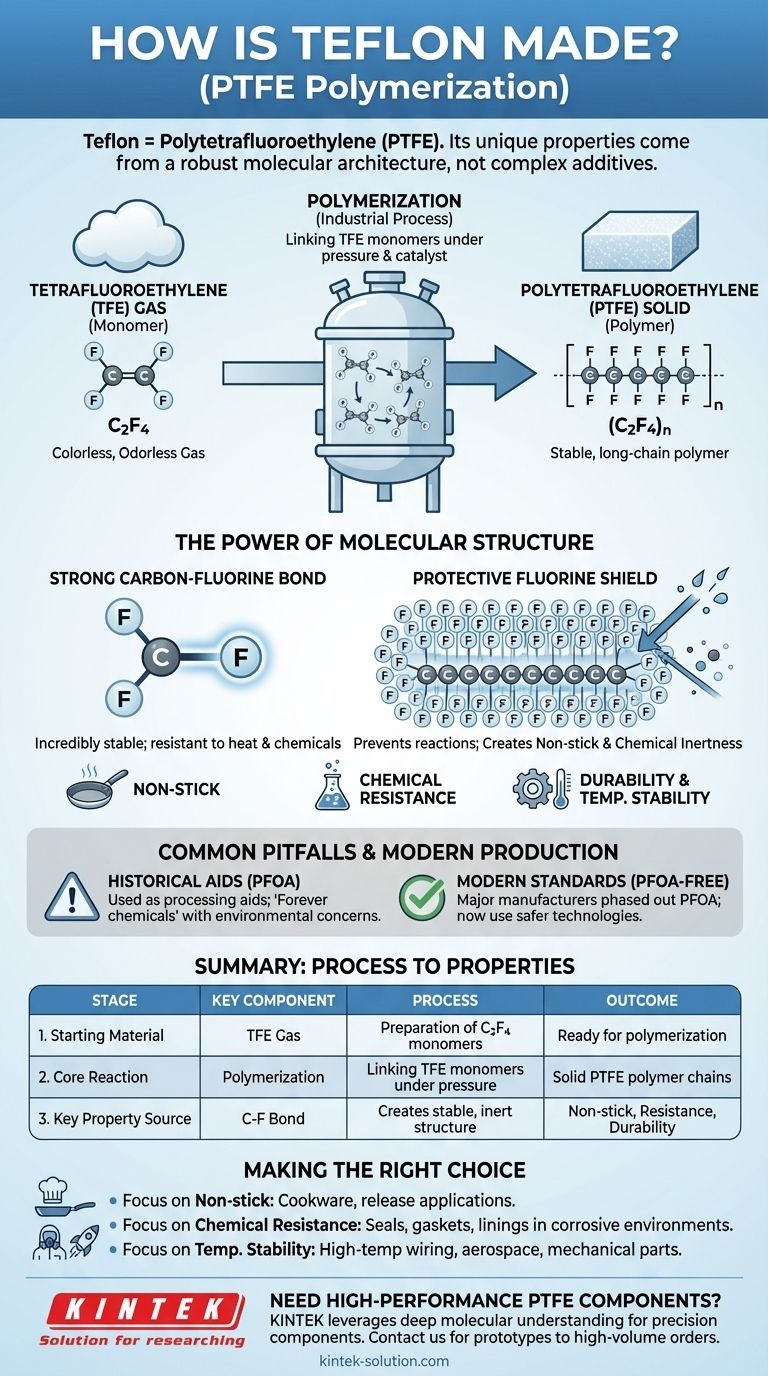
In essence, Teflon is the brand name for a polymer called Polytetrafluoroethylene (PTFE). It is created through a chemical process known as polymerization, where a gas called tetrafluoroethylene (TFE) is converted into a stable solid. The individual TFE molecules are linked together into extremely long, repeating chains, forming the robust material known for its unique properties.
The secret to Teflon's remarkable characteristics isn't a complex additive, but the fundamental strength of its molecular architecture. The powerful bond between carbon and fluorine atoms creates a highly stable, non-reactive structure that is responsible for its famous non-stick, chemical-resistant, and durable nature.

From Gas to Solid: The Core Process
The creation of PTFE is a fascinating example of how simple molecular building blocks can be assembled into a material with extraordinary capabilities. The process can be broken down into two primary stages.
The Starting Point: Tetrafluoroethylene (TFE)
The entire process begins with a colorless, odorless gas called tetrafluoroethylene (TFE).
Each molecule of TFE is composed of two carbon atoms and four fluorine atoms (C2F4). This small, individual molecule is known as a monomer.
The Key Step: Polymerization
The magic happens during polymerization. In this industrial process, vast quantities of TFE monomers are linked together end-to-end under specific conditions of pressure and in the presence of a catalyst.
This reaction transforms the simple gas into a solid, long-chain polymer. The resulting material is Polytetrafluoroethylene (PTFE), whose chemical formula is represented as (C2F4)n, where 'n' signifies a very large number of repeating monomer units.
Why This Structure Creates Teflon's Unique Properties
The manufacturing process is designed to create a specific molecular structure. This structure is the direct cause of the properties that make Teflon so valuable in both consumer and industrial applications.
The Power of the Carbon-Fluorine Bond
The bond between carbon and fluorine atoms is one of the strongest single bonds in organic chemistry. Because the entire PTFE molecule is built around this incredibly stable bond, it is exceptionally resistant to being broken down by heat or chemical attack.
A Protective Fluorine Shield
In the long PTFE chain, the carbon backbone is completely surrounded by a dense, tightly packed sheath of fluorine atoms.
This "fluorine shield" is crucial. It prevents other substances and chemicals from getting close enough to react with the carbon backbone, which is the source of Teflon's extreme chemical inertness and its famous non-stick surface.
The Impact on Durability
This molecular stability directly translates to physical toughness and a wide operating temperature range. The strong bonds don't break down easily, giving the material its signature durability, strength, and flexibility.
Common Pitfalls to Avoid
When discussing Teflon, it is critical to address the historical context of its manufacturing process to have a complete and objective understanding.
Historical Manufacturing Aids
For many decades, chemicals from a group called PFAS, most notably PFOA (perfluorooctanoic acid), were used as processing aids to help make PTFE.
These "forever chemicals" have raised significant environmental and health concerns because they do not easily break down and can accumulate over time.
Modern Production Standards
It is important to recognize that the industry has evolved. Major manufacturers, including the owner of the Teflon™ brand (Chemours), phased out the use of PFOA more than a decade ago. They have since transitioned to newer technologies with improved environmental and health profiles.
Making the Right Choice for Your Goal
Understanding the link between Teflon's creation and its properties helps clarify why it is chosen for specific jobs.
- If your primary focus is non-stick performance: The stable, low-friction fluorine shield is the reason it excels in cookware and other release applications.
- If your primary focus is chemical resistance: The same inert molecular structure makes PTFE the ideal choice for seals, gaskets, and linings in corrosive industrial environments.
- If your primary focus is temperature stability: The strength of the carbon-fluorine bond is what allows Teflon to be used in high-temperature wiring, aerospace applications, and demanding mechanical parts.
Ultimately, Teflon's creation process is designed to build a simple, immensely strong molecular chain that is the direct source of its extraordinary capabilities.
Summary Table:
| Stage | Key Component | Process | Outcome |
|---|---|---|---|
| 1. Starting Material | Tetrafluoroethylene (TFE) Gas | Preparation of C2F4 monomers | Ready for polymerization |
| 2. Core Reaction | Polymerization | Linking TFE monomers under pressure with a catalyst | Formation of solid PTFE polymer chains |
| 3. Key Property Source | Carbon-Fluorine Bond | Creates a stable, inert molecular structure | Non-stick, chemical resistance, durability |
Need high-performance PTFE components for your application?
At KINTEK, we leverage our deep understanding of PTFE's molecular science to manufacture precision components like seals, liners, and custom labware. Whether you're in the semiconductor, medical, laboratory, or industrial sector, our custom fabrication services—from prototypes to high-volume orders—ensure you get the exact chemical resistance, non-stick performance, and durability your project demands.
Contact our experts today to discuss your specific requirements and get a quote!
Visual Guide
Related Products
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
- Custom PTFE Bottles for Diverse Industrial Applications
People Also Ask
- How does PTFE react to common solvents? Discover Its Near-Total Chemical Immunity
- What is Teflon and what is its chemical name? Unpacking the Science of PTFE
- How was PTFE discovered and developed? From Lab Accident to Essential High-Performance Polymer
- What is the molecular structure of PTFE? The Key to Its Unmatched Chemical & Thermal Resistance
- What are the different types of Teflon available? A Guide to PTFE, FEP, PFA, and More



















