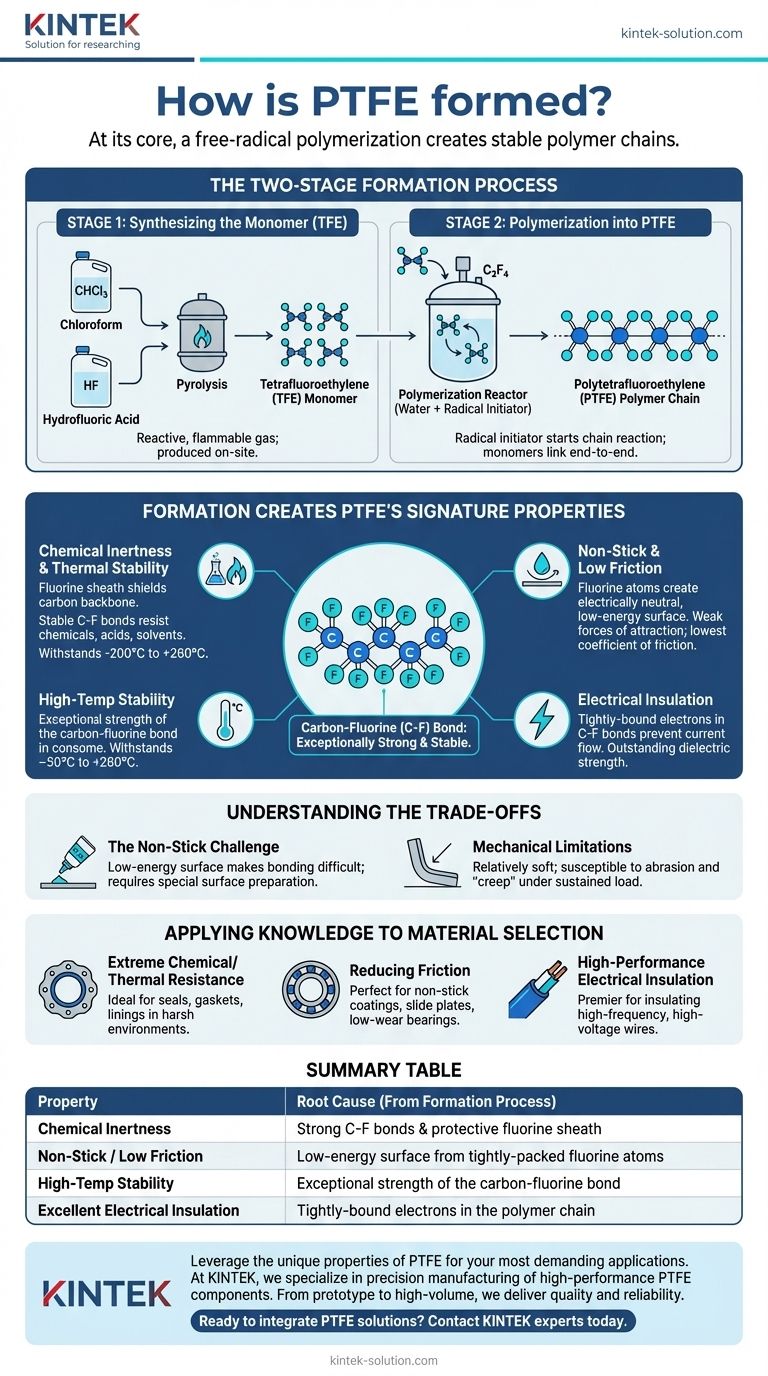
At its core, PTFE is formed through a process called free-radical polymerization. This chemical reaction takes a gas called tetrafluoroethylene (TFE) and links its individual molecules together into the long, incredibly stable polymer chains that constitute the final solid material we know as PTFE.
The creation of PTFE is a two-stage process that transforms an unstable, reactive gas into one of the most stable and inert materials ever engineered. This fundamental transformation is the direct source of its famous non-stick, chemical-resistant, and temperature-proof properties.

The Two-Stage Formation Process
The production of PTFE is not a simple, one-step reaction. It requires first creating the necessary building block—the monomer—and then assembling it into the final polymer.
Step 1: Synthesizing the Monomer (TFE)
Before PTFE can be made, its core component, tetrafluoroethylene (TFE), must be synthesized. This is typically done by reacting chloroform with hydrofluoric acid.
This process, known as pyrolysis, results in the TFE gas. Because TFE is highly reactive and flammable, it is almost always produced on-site, immediately before it is used in the next stage.
Step 2: Polymerization into PTFE
This is the crucial step where the material is actually formed. Molecules of TFE gas are passed through water containing a radical initiator under controlled pressure and temperature.
The initiator kicks off a chain reaction, causing the individual TFE molecules (monomers) to link together end-to-end. This process, free-radical polymerization, creates the long, repeating chains of polytetrafluoroethylene.
How Formation Creates PTFE's Signature Properties
The unique characteristics of PTFE are not magic; they are a direct result of the molecular structure created during polymerization. The process forges an exceptionally strong bond between carbon and fluorine atoms.
The Power of the Carbon-Fluorine Bond
The carbon-fluorine (C-F) bond is one of the strongest single bonds in organic chemistry. During polymerization, the carbon backbone of the polymer chain becomes completely encased in a protective sheath of fluorine atoms.
Explaining Chemical Inertness and Thermal Stability
This fluorine sheath effectively shields the carbon backbone from chemical attack. Because the C-F bonds are so stable and non-reactive, PTFE is inert to nearly all industrial chemicals, acids, and solvents. This same stability allows it to withstand an enormous temperature range, from –200°C to +260°C.
The Source of its "Slipperiness"
The fluorine atoms on the material's surface create an electrically neutral, low-energy surface with very weak forces of attraction. As a result, other substances have nothing to adhere to, which gives PTFE the lowest coefficient of friction of any known solid and its famous non-stick quality.
Understanding its Electrical Insulation
The electrons within the powerful carbon-fluorine bonds are held very tightly. This structure makes it extremely difficult for an electrical current to pass through the material, making PTFE an outstanding electrical insulator with high dielectric strength.
Understanding the Trade-offs
While its properties are remarkable, the very nature of PTFE's formation creates inherent limitations that are critical to understand for any application.
The Non-Stick Challenge
The same low-energy surface that makes PTFE non-stick also makes it extremely difficult to bond to other materials. Adhesives and glues simply can't get a grip on its "slippery" surface. Special processes like chemical etching are often required to prepare the surface for bonding.
Mechanical Limitations
While chemically robust, PTFE is a relatively soft material. Compared to metals or harder plastics, it can be more susceptible to abrasion and "creep"—the tendency to deform slowly under a sustained mechanical load.
Applying This Knowledge to Material Selection
Understanding the link between PTFE's formation and its properties allows you to select it with confidence for the right job.
- If your primary focus is extreme chemical resistance or temperature stability: The stable C-F bond structure makes PTFE the ideal choice for seals, gaskets, and linings in harsh chemical or thermal environments.
- If your primary focus is reducing friction: The unique fluorine sheath gives it an unmatched low coefficient of friction, perfect for non-stick coatings, slide plates, and low-wear bearings.
- If your primary focus is high-performance electrical insulation: Its tightly-bound electrons make it a premier material for insulating high-frequency and high-voltage wires and cables.
By understanding how PTFE is made, you can see that its extraordinary properties are a direct and predictable result of its fundamental chemical structure.
Summary Table:
| Property | Root Cause (From Formation Process) |
|---|---|
| Chemical Inertness | Strong C-F bonds & protective fluorine sheath |
| Non-Stick / Low Friction | Low-energy surface from tightly-packed fluorine atoms |
| High-Temp Stability | Exceptional strength of the carbon-fluorine bond |
| Excellent Electrical Insulation | Tightly-bound electrons in the polymer chain |
Leverage the unique properties of PTFE for your most demanding applications. At KINTEK, we specialize in the precision manufacturing of high-performance PTFE components—from custom seals and liners to complex labware. Our expertise ensures your designs benefit from PTFE's full potential, whether for semiconductor, medical, laboratory, or industrial use. From prototype to high-volume production, we deliver the quality and reliability your projects require.
Ready to integrate PTFE solutions into your next project? Contact our experts today to discuss your specific needs.
Visual Guide
Related Products
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
- Custom PTFE Bottles for Diverse Industrial Applications
People Also Ask
- How does PTFE's molecular structure contribute to its non-stick properties? The Science Behind Its Slick Surface
- What is PTFE commonly known as and what type of material is it? A Guide to High-Performance PTFE Properties
- What is Teflon and what is its chemical name? Unpacking the Science of PTFE
- How was PTFE discovered and developed? From Lab Accident to Essential High-Performance Polymer
- How does PTFE react to common solvents? Discover Its Near-Total Chemical Immunity



















