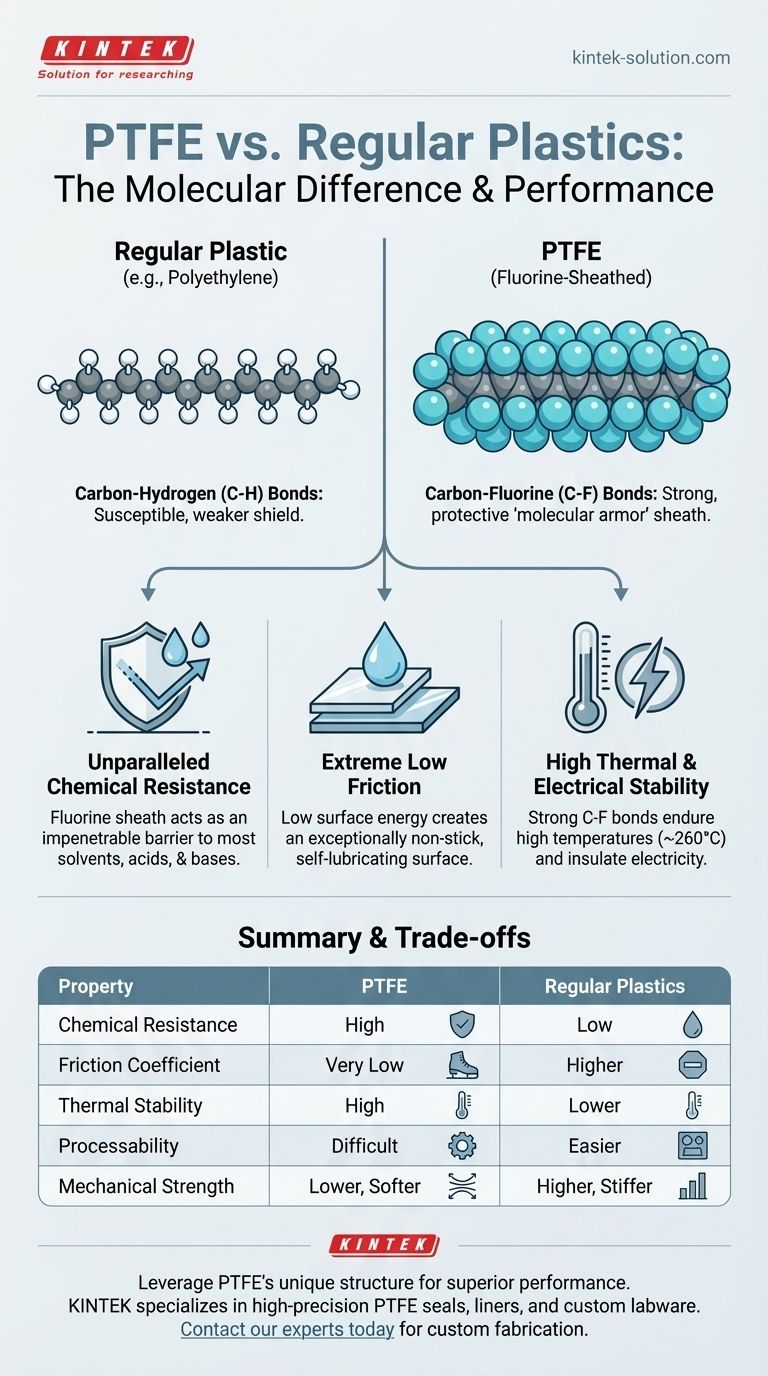
At a molecular level, the fundamental difference between Polytetrafluoroethylene (PTFE) and regular plastics is the substitution of atoms. While common plastics like polyethylene are built on a carbon backbone with attached hydrogen atoms, PTFE’s structure replaces every hydrogen atom with a larger fluorine atom, creating a material with radically different properties.
The core distinction is that fluorine atoms form a tight, protective sheath around PTFE's carbon backbone. This molecular armor is the source of its exceptional chemical inertness, low friction, and thermal stability, setting it apart from virtually all other polymers.

The Foundation: A Carbon Backbone Sheathed in Fluorine
All plastics are polymers, meaning they are long chains of repeating molecular units. The critical difference in PTFE lies in what is attached to its primary chain.
The Polymer Chain
Like many common plastics, PTFE starts with a long, linear chain of carbon atoms. This carbon-carbon bond forms the structural backbone of the material.
The Protective Fluorine Sheath
In PTFE, every available bonding spot on the carbon chain is occupied by a fluorine atom. Because fluorine atoms are significantly larger than hydrogen atoms, they twist around the backbone, forming a dense, uniform, and continuous protective shell. This "fluorine sheath" is the key to all of PTFE's signature characteristics.
The Carbon-Fluorine Bond
The bond between carbon and fluorine is exceptionally strong and stable. This powerful bond, repeated millions of times along the polymer chain, contributes significantly to PTFE's overall robustness. It requires a tremendous amount of energy to break.
How Structure Dictates Unmatched Performance
The unique molecular arrangement of PTFE directly translates into a set of properties that are extreme even among high-performance plastics.
Unparalleled Chemical Resistance
The fluorine sheath acts as an impenetrable barrier. It physically and chemically protects the vulnerable carbon backbone from attack, making PTFE virtually impervious to almost all industrial solvents, acids, and bases. This level of inertness outperforms other strong plastics like PEEK and Nylon.
Extreme Low Friction
The fluorine sheath also creates an incredibly low surface energy. The fluorine atoms are held so tightly that they generate very weak forces of attraction with other molecules. This molecular "indifference" is what makes PTFE's surface exceptionally non-stick and low-friction.
High Thermal and Electrical Stability
The strength of the carbon-fluorine bond gives PTFE a very high melting point and allows it to remain stable across a wide range of temperatures. Furthermore, the uniform, symmetrical structure of the sheathed molecule makes it an excellent electrical insulator.
Understanding the Trade-offs
No material is perfect, and the molecular structure that gives PTFE its incredible strengths also creates inherent limitations.
Processing Challenges
The same chemical inertness and high melt viscosity that make PTFE so durable also make it very difficult to process. It cannot be melted and injection-molded like common plastics. Instead, it requires specialized and often more costly techniques like sintering.
Lower Mechanical Strength
While incredibly stable, PTFE is a relatively soft material. Compared to engineering plastics like Nylon, which has amide bonds that provide strength, PTFE has lower tensile strength and is more susceptible to creep (slow deformation under sustained pressure).
Making the Right Choice for Your Goal
Understanding the molecular difference allows you to select a material based on its fundamental capabilities.
- If your primary focus is extreme chemical inertness or the lowest possible friction: PTFE's fluorine-sheathed structure is the definitive choice.
- If your primary focus is high mechanical strength, stiffness, or ease of manufacturing: A polymer like Nylon, with a different molecular bond structure, will likely be a more suitable and cost-effective solution.
Ultimately, recognizing how a simple atomic substitution creates a molecular sheath is key to leveraging PTFE's power correctly.
Summary Table:
| Property | PTFE (Fluorine-Sheathed) | Regular Plastics (e.g., Polyethylene) |
|---|---|---|
| Chemical Resistance | Extremely high, inert to most chemicals | Low to moderate, susceptible to solvents |
| Friction Coefficient | Exceptionally low (non-stick) | Higher |
| Thermal Stability | High (operates up to ~260°C / 500°F) | Lower |
| Primary Atomic Backbone | Carbon-Fluorine (C-F) bonds | Carbon-Hydrogen (C-H) bonds |
| Processability | Difficult, requires sintering | Easier, often injection-molded |
| Mechanical Strength | Lower tensile strength, softer | Generally higher strength and stiffness |
Need a PTFE component that leverages this unique molecular structure for superior performance?
At KINTEK, we specialize in manufacturing high-precision PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors. Our expertise ensures your parts deliver the unmatched chemical inertness, low friction, and thermal stability that only PTFE can provide.
Contact our experts today to discuss your custom fabrication needs, from prototypes to high-volume orders.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Customizable PTFE Rods for Advanced Industrial Applications
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
People Also Ask
- What are the non-stick properties of PTFE and their applications? Unlock Clean Release & Low Friction
- What is the relationship between PTFE and Teflon? A Guide to the Material vs. the Brand Name
- How does Teflon's low surface energy contribute to its properties? The Science Behind Non-Stick & Low Friction
- How does PTFE perform under high temperatures? Leverage Its Exceptional Thermal Stability Up to 260°C
- What are the primary applications of PTFE in electrical and aerospace industries? Ensure Reliability in Extreme Environments
- What is PTFE and what are its key attributes? The Ultimate Guide to Its Properties & Uses
- What forms does Teflon come in? A Guide to PTFE States, Formulations, and Applications
- What are some common industrial applications of PTFE? Essential for Extreme Environments



















