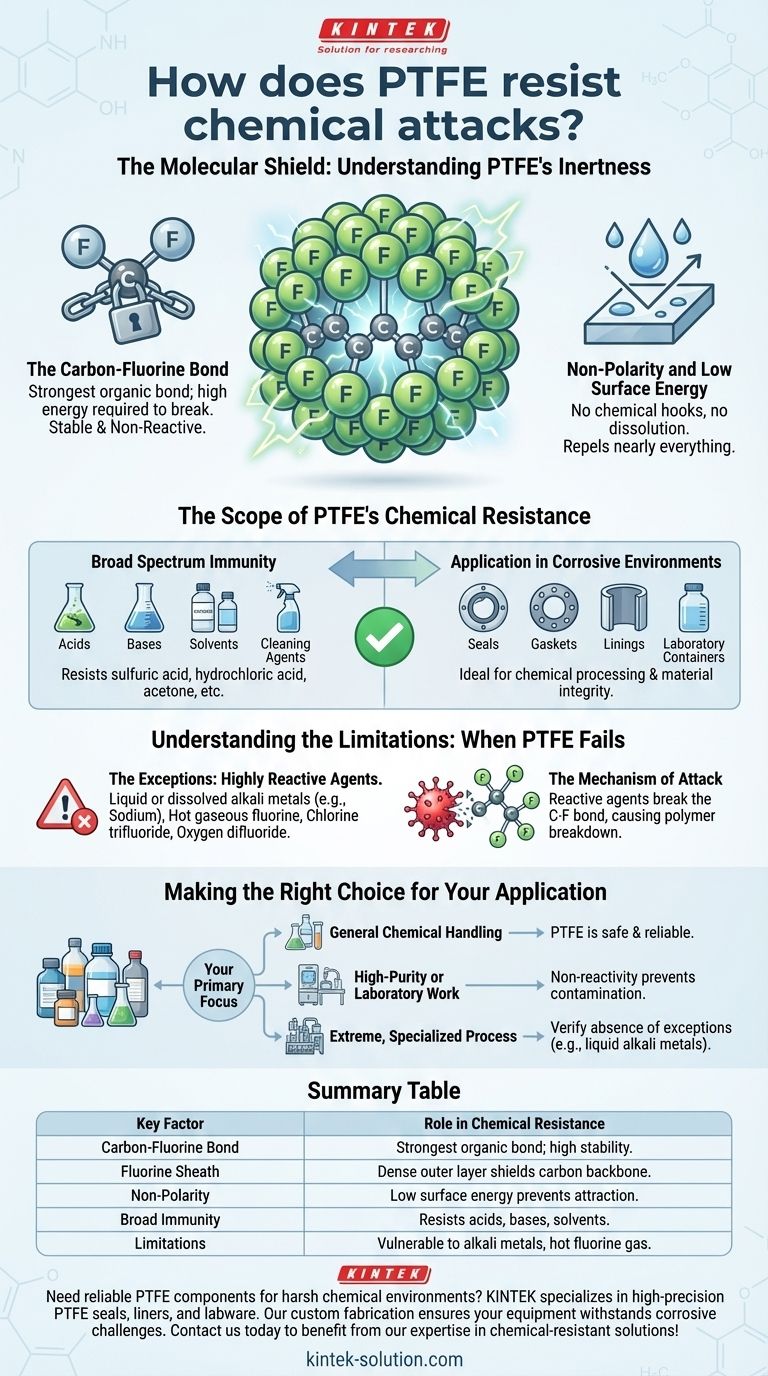
At its core, PTFE's remarkable chemical resistance comes from its molecular structure. The exceptionally strong bonds between its carbon and fluorine atoms create a stable, non-reactive material that acts as a fortress against nearly all chemical attacks.
PTFE's chemical inertness is not a complex feature but a direct result of its simple, powerful carbon-fluorine bonds. This makes it a default material for corrosive environments, but you must be aware of the few, highly specific chemicals that can breach its defenses.

The Molecular Shield: Understanding PTFE's Inertness
Polytetrafluoroethylene (PTFE) is virtually immune to chemical attack because its fundamental design leaves no opening for other chemicals to react with it. This inertness is a product of its atomic composition and structure.
The Carbon-Fluorine Bond
The bond between carbon and fluorine atoms is one of the strongest single bonds in organic chemistry. This immense strength means it takes an extraordinary amount of energy to break, making the molecule highly stable and non-reactive.
Think of the molecule's carbon backbone as being protected by a perfect, tightly-knit suit of armor made of fluorine atoms.
The Fluorine Sheath
The fluorine atoms are relatively large and are packed very tightly around the central carbon chain. This dense outer layer of fluorine atoms effectively shields the vulnerable carbon backbone from any potential chemical interaction.
This physical barrier prevents even aggressive chemicals from getting close enough to initiate a reaction.
Non-Polarity and Low Surface Energy
PTFE is a non-polar molecule with very low surface energy. This means it has no "chemical hooks" to attract other molecules, which is why it does not dissolve in any known solvent at room temperature and repels nearly everything it contacts.
The Scope of PTFE's Chemical Resistance
The practical result of this molecular structure is a material that remains stable and intact when exposed to a vast range of corrosive and aggressive substances.
Broad Spectrum Immunity
PTFE demonstrates exceptional resistance to a wide array of chemicals. This includes common industrial substances like acids, bases, solvents, and cleaning agents such as chlorine dioxide.
It remains unaffected by substances like sulfuric acid, hydrochloric acid, acetone, and sodium peroxide, which would degrade most other materials.
Application in Corrosive Environments
This robust chemical resistance makes PTFE an ideal material for demanding applications. It is frequently used for seals, gaskets, linings for chemical processing equipment, and laboratory containers where material integrity is critical.
Its use ensures that the equipment will not degrade and that the chemicals being handled will not be contaminated.
Understanding the Limitations: When PTFE Fails
While its resistance is vast, PTFE is not invincible. A handful of extremely reactive chemicals can attack and degrade the material under specific conditions.
The Exceptions: Highly Reactive Agents
PTFE's defenses can be breached by a very short list of substances. These include liquid or dissolved alkali metals (like sodium), hot gaseous fluorine, and other potent fluorinating agents like chlorine trifluoride and oxygen difluoride.
The Mechanism of Attack
These specific chemicals are among the few substances reactive enough to overcome the strength of the carbon-fluorine bond. They have the ability to strip the fluorine atoms from the carbon backbone, causing the polymer to break down.
It is crucial to note that these are highly specialized and dangerous chemicals not found in the vast majority of industrial or commercial applications.
Making the Right Choice for Your Application
Understanding both the profound strengths and the specific, narrow weaknesses of PTFE is key to using it effectively and safely.
- If your primary focus is general chemical handling: PTFE is an exceptionally safe and reliable choice for environments involving nearly all common acids, bases, and organic solvents.
- If your primary focus is high-purity or laboratory work: PTFE's non-reactivity is ideal, as it prevents leaching and contamination of sensitive materials.
- If your primary focus is an extreme, specialized process: You must verify that your environment does not contain the few exceptions, such as liquid alkali metals or potent fluorinating gases.
By knowing the precise boundaries of its capabilities, you can confidently leverage PTFE's unparalleled chemical resistance for your project.
Summary Table:
| Key Factor | Role in Chemical Resistance |
|---|---|
| Carbon-Fluorine Bond | One of the strongest bonds in organic chemistry, providing high stability. |
| Fluorine Sheath | Dense outer layer of fluorine atoms shields the carbon backbone from attack. |
| Non-Polarity | Low surface energy prevents chemical attraction and dissolution. |
| Broad Immunity | Resists acids, bases, solvents (e.g., sulfuric acid, acetone). |
| Limitations | Vulnerable to alkali metals, hot fluorine gas, and fluorinating agents. |
Need reliable PTFE components for harsh chemical environments? KINTEK specializes in manufacturing high-precision PTFE seals, liners, and labware for semiconductor, medical, laboratory, and industrial applications. Our custom fabrication services—from prototypes to high-volume orders—ensure your equipment withstands corrosive challenges. Contact us today to discuss your specific needs and benefit from our expertise in chemical-resistant solutions!
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- What are the key structural components of Teflon? Unlocking the Secrets of PTFE's Performance
- What forms is PTFE available in besides sheets and rods? Discover the Full Range of PTFE Options
- What are the key physical and chemical properties of PTFE? Unlock Unmatched Chemical & Thermal Resistance
- What is creep and how does it affect PTFE? Ensure Long-Term Reliability for Your Components
- What is PTFE and what is it commonly known as? The Ultimate Guide to Teflon & Its Uses
- How does the electronegativity of fluorine affect PTFE's structure? The Key to Its Unmatched Chemical Resistance
- What are the limitations of PTFE as a material? Key Mechanical Weaknesses to Consider
- What are the advantages of expanded PTFE (ePTFE)? Unlock Superior Sealing and Flexibility



















