In pharmaceutical manufacturing, PTFE is indispensable for its unique combination of chemical inertness, sterility, and durability. It is used in critical components like gaskets, valves, and coatings to ensure product purity, prevent contamination, and maintain process integrity in highly regulated environments. This makes Polytetrafluoroethylene (PTFE) a cornerstone material for producing safe and effective medicines.
The core challenge in pharmaceutical manufacturing is eliminating any risk of contamination while maximizing operational efficiency. PTFE directly addresses this by providing a non-reactive, sterile, and long-lasting material for equipment that comes into contact with sensitive compounds, cleaning agents, and high-temperature sterilization processes.

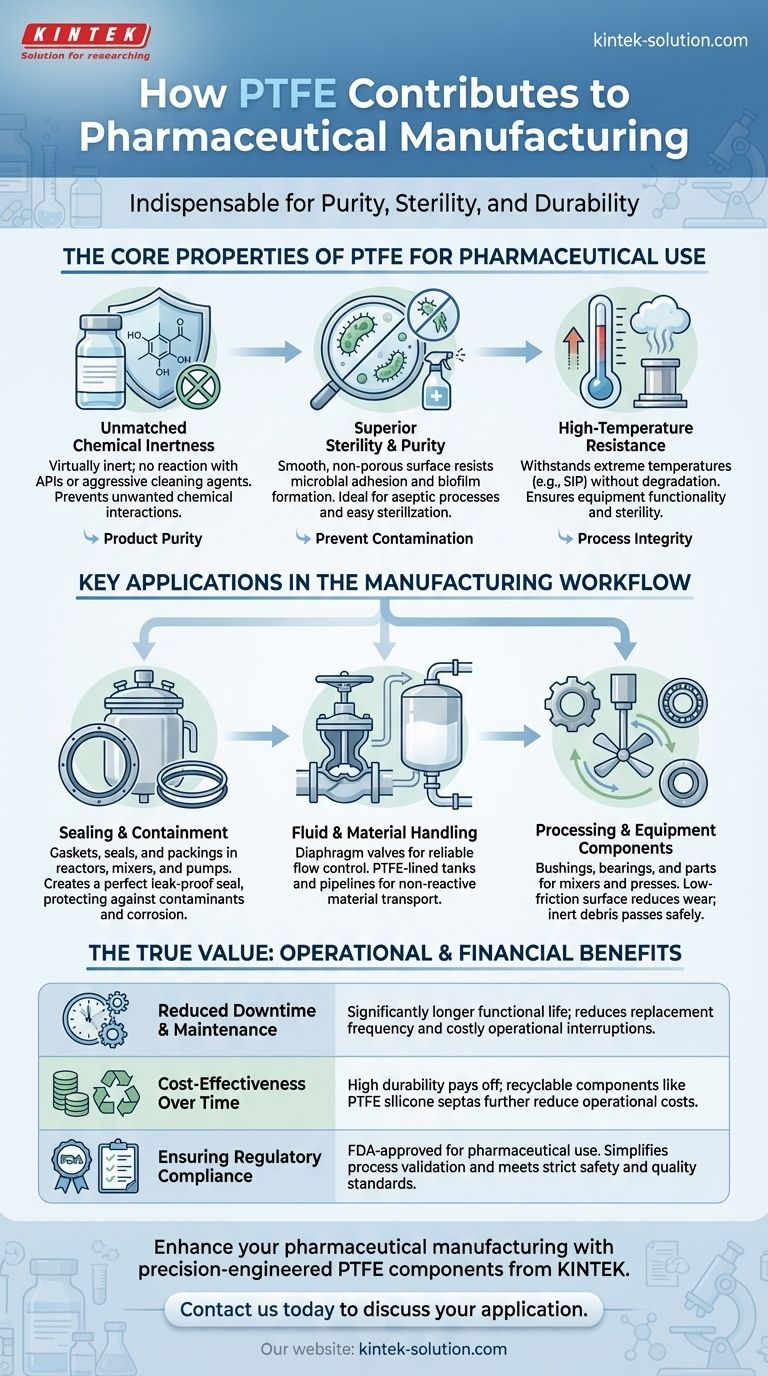
The Core Properties of PTFE for Pharmaceutical Use
To understand PTFE's role, we must first examine its fundamental properties. These characteristics are not just beneficial; they are often mandatory to meet the industry's stringent standards.
Unmatched Chemical Inertness
PTFE is virtually inert, meaning it does not react with pharmaceutical ingredients, active pharmaceutical ingredients (APIs), or aggressive cleaning agents. This non-reactive nature is critical for preventing unwanted chemical interactions that could compromise the final product's purity and safety.
Superior Sterility and Purity
The material's exceptionally smooth, non-porous surface resists microbial adhesion and biofilm formation. This makes PTFE surfaces easy to clean and sterilize, creating an ideal environment for aseptic processes where preventing contamination is paramount.
High-Temperature Resistance
Pharmaceutical manufacturing often involves high-temperature sterilization methods like steam-in-place (SIP). PTFE components, such as expansion bellows and valve diaphragms, can withstand these extreme temperatures without degrading, ensuring equipment remains functional and sterile.
Key Applications in the Manufacturing Workflow
PTFE is not a single-use material; it is integrated across the entire production line in various forms, each solving a specific problem.
Sealing and Containment
PTFE is widely used for gaskets, seals, and packings in equipment such as reactors, mixers, and pumps. These components create a perfect seal that prevents leaks and protects the product from external contaminants while resisting corrosion from strong oxidizers or cleaning fluids.
Fluid and Material Handling
In systems that transport liquids and other materials, PTFE is crucial. Diaphragm valves made with PTFE offer a high cycle life, ensuring reliable flow control. PTFE-lined tanks and pipelines provide a non-reactive barrier, protecting both the product and the equipment itself from chemical corrosion.
Processing and Equipment Components
Within machinery, PTFE serves as bushings, bearings, and parts for mixers and presses. Its low-friction surface reduces wear and tear. Importantly, any microscopic debris from these seals is inert and can pass through the human body without consequence, adding a layer of safety.
The True Value: Operational and Financial Benefits
Beyond its technical properties, the adoption of PTFE provides tangible advantages that impact a facility's bottom line and regulatory standing.
Reduced Downtime and Maintenance
Components like PTFE diaphragm valves and bellows have a significantly longer functional life than those made from other materials. This high durability reduces the frequency of replacement, minimizing costly operational downtime and lowering annual maintenance expenses.
Cost-Effectiveness Over Time
While the initial investment may be higher, the longevity and reliability of PTFE pay dividends. Furthermore, certain components like PTFE silicone septas are recyclable, which helps reduce recurring operational costs without compromising quality or performance.
Ensuring Regulatory Compliance
PTFE is approved by regulatory bodies like the FDA for use in pharmaceutical applications. Using compliant materials from the outset simplifies process validation and ensures that manufacturing workflows meet the industry's strict safety and quality standards.
How to Apply This to Your Process
Choosing the right material is a critical decision. Your primary operational goal should guide your material specification.
- If your primary focus is ensuring absolute product purity: Prioritize PTFE for any surface that comes into contact with the product, such as gaskets, tank linings, and pump diaphragms.
- If your primary focus is maximizing operational uptime: Invest in high-cycle-life PTFE components like diaphragm valves to reduce maintenance intervals and prevent unexpected failures.
- If your primary focus is meeting stringent regulatory standards: Ensure all product-contact materials, especially seals and packings, are made from FDA-approved grades of PTFE.
Ultimately, integrating PTFE into pharmaceutical manufacturing is a strategic decision to safeguard product integrity, enhance operational reliability, and ensure compliance.
Summary Table:
| Key Property | Benefit in Pharma Manufacturing |
|---|---|
| Chemical Inertness | Prevents reaction with APIs and cleaning agents |
| Superior Sterility | Resists microbial adhesion, easy to sterilize |
| High-Temperature Resistance | Withstands SIP and other sterilization processes |
| Durability | Reduces downtime and maintenance costs |
Enhance your pharmaceutical manufacturing with precision-engineered PTFE components from KINTEK. Our PTFE seals, liners, labware, and custom-fabricated parts are designed to meet the stringent demands of the semiconductor, medical, laboratory, and industrial sectors. By prioritizing precision production and offering custom solutions from prototypes to high-volume orders, we help you ensure product purity, maximize operational uptime, and achieve regulatory compliance. Contact us today (#ContactForm) to discuss how our PTFE solutions can benefit your specific application.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Customizable PTFE Rods for Advanced Industrial Applications
People Also Ask
- What finishing techniques are effective for machined Teflon parts? Achieve Functional Performance and Dimensional Stability
- What are the unique properties of PTFE? The 3 Pillars Driving Demand for High-Performance Parts
- What factors should be considered when choosing between Nylon and PTFE? Select the Right Material for Your Application
- What are the unique properties of PTFE? Unlock Unmatched Performance in Demanding Applications
- What industrial benefits do PTFE-machined parts offer? Achieve Peak Performance in Demanding Applications



















