In pharmaceutical analytics, PTFE/silicone septas enhance efficiency by creating a chemically inert and physically reliable barrier that preserves sample integrity. This barrier prevents costly contamination, ensures sample stability during storage and testing, and enables the consistent performance of automated, high-throughput analytical instruments.
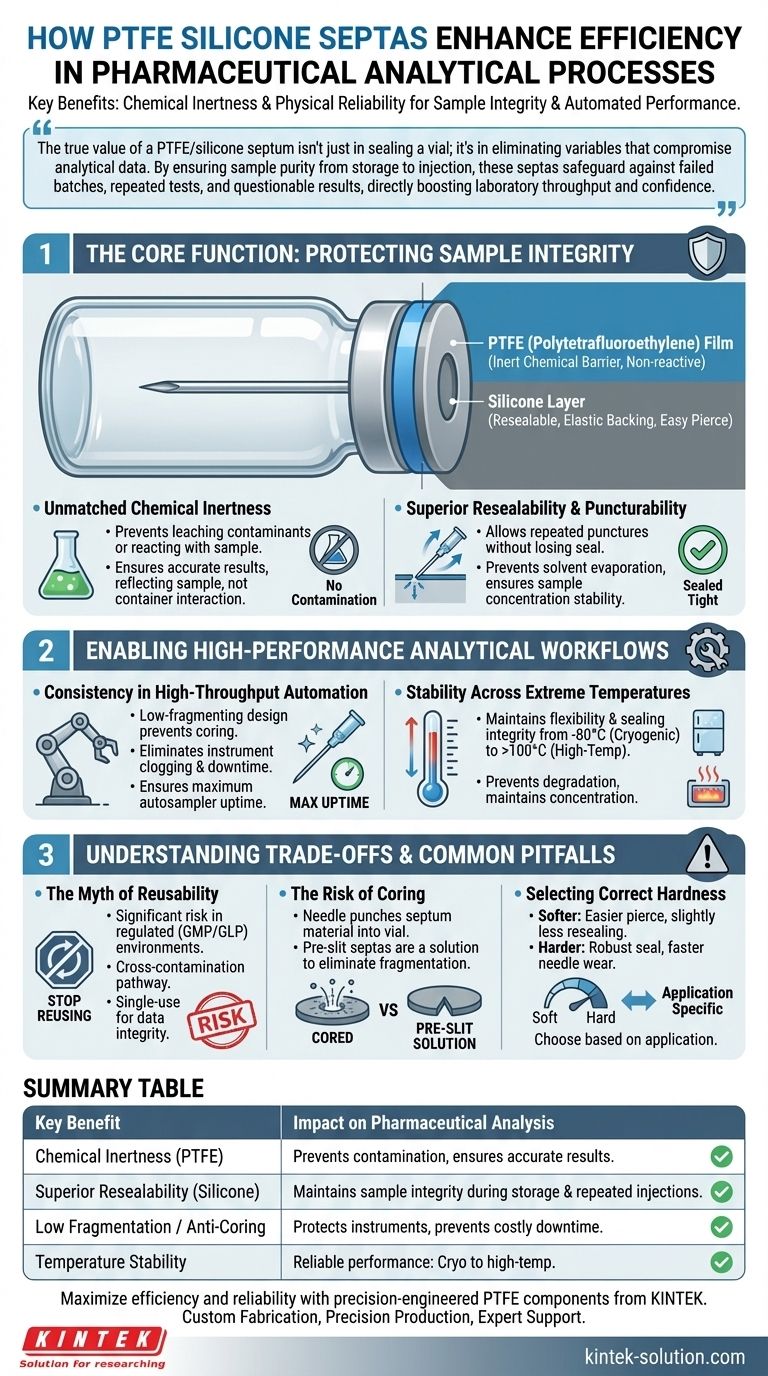
The true value of a PTFE/silicone septum isn't just in sealing a vial; it's in eliminating variables that compromise analytical data. By ensuring sample purity from storage to injection, these septas safeguard against failed batches, repeated tests, and questionable results, directly boosting laboratory throughput and confidence.

The Core Function: Protecting Sample Integrity
The primary role of a septum is to protect the sample from the outside world and itself. PTFE/silicone composites are engineered to excel at this task through a dual-material design.
The Power of a Dual-Layer Design
A PTFE/silicone septum combines two materials, each with a distinct purpose. The PTFE (polytetrafluoroethylene) film faces the sample, providing an almost universally inert chemical barrier.
The thicker silicone layer is positioned behind the PTFE. It serves as a soft, elastic backing that allows a needle to pierce it easily and, crucially, reseals after the needle is withdrawn.
Unmatched Chemical Inertness
In pharmaceutical analysis, samples can range from highly acidic or alkaline solutions to aggressive organic solvents. The PTFE layer is non-reactive, preventing it from leaching contaminants into the drug formulation or reacting with the sample itself.
This inertness ensures that the analytical results reflect the sample, not an interaction with its container, which is a foundational requirement for accurate and reliable data.
Superior Resealability and Puncturability
The silicone layer provides the mechanical efficiency. Its elasticity allows for repeated punctures from autosampler needles without losing its sealing capability.
This is critical for workflows where a sample might be analyzed multiple times or left on an autosampler tray for an extended period. A proper seal prevents solvent evaporation, which would otherwise concentrate the sample and produce falsely high readings.
Enabling High-Performance Analytical Workflows
Efficiency in a modern lab is tied directly to automation and reliability. PTFE/silicone septas are a small but critical component in maintaining the pace and quality of these workflows.
Consistency in High-Throughput Automation
Automated systems like HPLC and GC autosamplers rely on predictable, repeatable actions. A low-quality septum can fragment or "core" when punctured, depositing tiny particles into the sample vial.
These particles can clog the delicate tubing and needles of the instrument, leading to sequence failures, system downtime, and expensive repairs. High-quality PTFE/silicone septas are designed to be low-fragmenting, ensuring maximum autosampler uptime.
Stability Across Extreme Temperatures
Pharmaceutical research involves a wide range of temperature conditions. This includes high-temperature drug stability studies and the cryogenic storage of sensitive biological samples.
PTFE/silicone septas maintain their flexibility and sealing integrity across this broad temperature spectrum. This ensures that a sample thawed from -80°C or heated to over 100°C remains perfectly sealed, preventing degradation and maintaining its original concentration.
Understanding the Trade-offs and Common Pitfalls
While highly effective, not all PTFE/silicone septas are created equal, and their application requires an understanding of their limitations.
The Myth of Reusability in Critical Applications
Some sources mention reusability as a cost-saving benefit. While technically possible, reusing septas in a regulated pharmaceutical environment (GMP/GLP) is a significant risk.
Each puncture creates a potential pathway for cross-contamination between samples. For any work that requires unimpeachable data integrity, septas must be considered a single-use consumable. The cost of a failed batch far outweighs the savings from reusing septas.
The Risk of Coring
Coring occurs when the needle punches a small piece of the septum material into the vial. While quality septas are designed to minimize this, it can still happen, especially with dull needles or improperly matched septa.
Pre-slit septas are an excellent solution to this problem. The slit guides the needle through the septum, virtually eliminating fragmentation and the risk of coring.
Selecting the Correct Hardness (Durometer)
Silicone is available in different hardness levels, or durometers. A softer septum is easier for a needle to pierce but may have slightly less effective resealing properties. A harder septum provides a more robust seal but can cause faster wear on autosampler needles. The choice depends on the specific application and instrumentation.
Making the Right Choice for Your Goal
Selecting the correct septum is a strategic decision that supports your specific analytical objective.
- If your primary focus is regulatory compliance (GMP/GLP): Prioritize single-use, certified high-purity septas to eliminate any risk of cross-contamination and ensure data defensibility.
- If your primary focus is high-throughput screening: Select pre-slit septas optimized for low fragmentation to ensure maximum autosampler uptime and prevent failed runs.
- If your primary focus is volatile compound analysis: Use non-slit, high-quality septas to provide the most secure seal against evaporation over long analytical sequences.
Ultimately, selecting the correct septum is a foundational decision that directly impacts the speed, cost, and reliability of your analytical outcomes.
Summary Table:
| Key Benefit | Impact on Pharmaceutical Analysis |
|---|---|
| Chemical Inertness (PTFE Layer) | Prevents sample contamination and reaction, ensuring accurate analytical results. |
| Superior Resealability (Silicone Layer) | Maintains sample integrity during storage and repeated autosampler injections. |
| Low Fragmentation / Anti-Coring | Protects sensitive instrumentation, preventing costly downtime and repairs. |
| Temperature Stability | Reliable performance from cryogenic storage to high-temperature stability studies. |
Maximize the efficiency and reliability of your pharmaceutical analysis with precision-engineered PTFE components from KINTEK.
Our PTFE/silicone septas are manufactured to the highest standards of purity and performance, directly supporting the demanding needs of semiconductor, medical, laboratory, and industrial applications. We understand that your success depends on uncompromised data integrity and maximum instrument uptime.
Let us provide the solution you need:
- Custom Fabrication: From prototype to high-volume orders, we tailor components to your exact specifications.
- Precision Production: Consistent, high-quality parts that integrate seamlessly into your automated workflows.
- Expert Support: Technical expertise to help you select the optimal septum for your specific application (e.g., GMP/GLP compliance, high-throughput screening).
Ready to enhance your lab's performance? Contact KINTEK today to discuss your requirements and request a quote.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- PTFE Chemical Solvent Sampling Spoon
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Customizable PTFE Seals Filter Holders for Versatile Applications
People Also Ask
- Why are PTFE lined vials considered durable? Superior Chemical & Thermal Resistance for Reliable Performance
- Why are PTFE filters advantageous for gravimetric analysis? Achieve Unmatched Accuracy and Precision
- What are the features of PTFE laboratory bottles? Unmatched Chemical Resistance & Extreme Temperature Tolerance
- How do the costs of PTFE and silicone septa compare? Understand the Value Beyond Price
- What is the significance of thickness in PTFE-coated septums? Maximize Durability and Analytical Reliability
- What factors should be considered when choosing between PTFE and silicone septa? Ensure Chemical Compatibility and Reliable Sealing
- Why are PTFE shovels considered cost-effective? Maximize ROI with Superior Durability
- What factors should be considered when selecting a PTFE shovel for laboratory use? Ensure Chemical Inertness & Sample Integrity



















