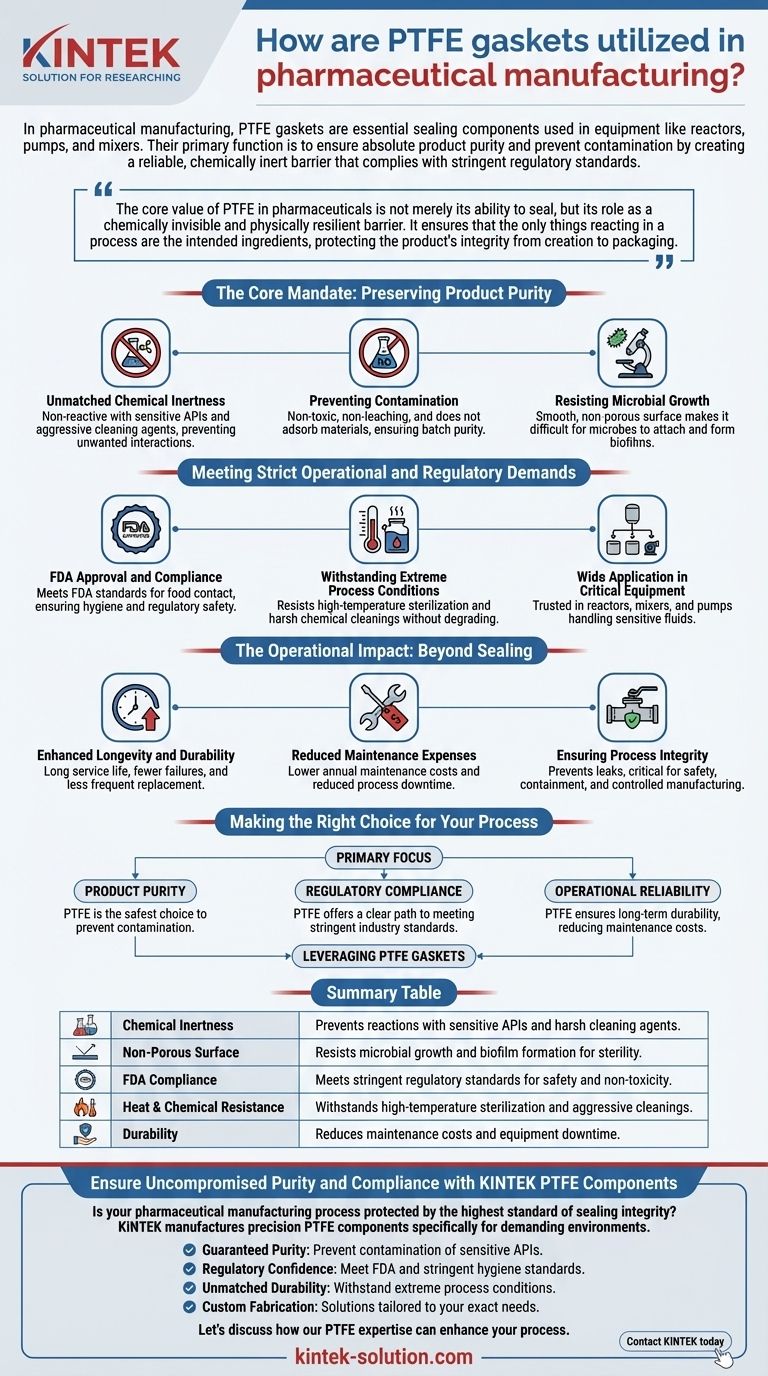
In pharmaceutical manufacturing, PTFE gaskets are essential sealing components used in equipment like reactors, pumps, and mixers. Their primary function is to ensure absolute product purity and prevent contamination by creating a reliable, chemically inert barrier that complies with stringent regulatory standards.
The core value of PTFE in pharmaceuticals is not merely its ability to seal, but its role as a chemically invisible and physically resilient barrier. It ensures that the only things reacting in a process are the intended ingredients, protecting the product's integrity from creation to packaging.

The Core Mandate: Preserving Product Purity
In pharmaceutical production, even the slightest contamination or unintended chemical reaction can compromise the safety and efficacy of a drug. PTFE is specifically chosen for its unique combination of properties that directly address this core challenge.
Unmatched Chemical Inertness
PTFE is virtually non-reactive with the vast majority of chemicals. This means it will not react with sensitive active pharmaceutical ingredients (APIs) or the aggressive cleaning agents and strong oxidizers used to sterilize equipment.
This inertness is critical for preventing unwanted chemical interactions that could create impurities or degrade the final product.
Preventing Contamination
PTFE gaskets are non-toxic and do not leach or adsorb materials. They will not release any of their own components into the process fluid, nor will they absorb the product.
This ensures that each batch of medicine is pure and maintains its precise chemical composition without interference from the sealing material.
Resisting Microbial Growth
The material possesses a very smooth, non-porous surface. This physical characteristic makes it difficult for bacteria and other microbes to attach and form biofilms.
In a sterile manufacturing environment, this resistance to microbial growth is a fundamental requirement for preventing contamination and ensuring product safety.
Meeting Strict Operational and Regulatory Demands
Pharmaceutical manufacturing is governed by strict regulations and operates under demanding conditions. Gasket materials must perform reliably while meeting all compliance requirements.
FDA Approval and Compliance
PTFE is approved by the FDA for use in applications involving food contact. This same standard is often leveraged in the pharmaceutical industry as a benchmark for safety and non-toxicity.
Using an FDA-compliant material like PTFE helps manufacturers meet hygiene standards and fulfill their regulatory obligations with confidence.
Withstanding Extreme Process Conditions
Pharmaceutical equipment is frequently subjected to high-temperature sterilization cycles and harsh chemical cleanings.
PTFE components, such as expansion bellows and gaskets, are engineered to withstand these conditions without degrading. This resilience ensures they maintain a perfect seal and do not break down over time.
Wide Application in Critical Equipment
Due to these properties, PTFE gaskets and packings are trusted components in the most critical parts of the production line.
You will find them in reactors where APIs are synthesized, mixers where ingredients are blended, and pumps that transfer sensitive fluids throughout the facility.
The Operational Impact: Beyond Sealing
The choice of PTFE provides tangible benefits that extend beyond basic compliance and sealing, impacting the efficiency and cost-effectiveness of the entire operation.
Enhanced Longevity and Durability
Because PTFE does not degrade when exposed to heat or aggressive chemicals, gaskets made from it have a long service life. This durability means fewer failures and less frequent replacement.
Reduced Maintenance Expenses
The longevity of PTFE components directly translates to lower annual maintenance costs. Less time and money are spent on replacing worn-out gaskets, which also reduces process downtime.
Ensuring Process Integrity
Reliable sealing prevents fluid leakage, which is critical for both safety and efficiency. By maintaining a perfect seal under pressure and temperature, PTFE gaskets ensure the entire manufacturing process remains contained and controlled.
Making the Right Choice for Your Process
Selecting the correct gasket material is a foundational decision in pharmaceutical equipment design, directly influencing product quality, safety, and operational stability.
- If your primary focus is product purity: PTFE's chemical inertness and non-leaching properties make it the safest choice to prevent contamination and unwanted reactions.
- If your primary focus is regulatory compliance: The established FDA-approved status of PTFE provides a clear and reliable path to meeting stringent industry standards.
- If your primary focus is operational reliability: PTFE's resistance to high temperatures and harsh chemicals ensures long-term durability, reducing maintenance costs and equipment downtime.
Ultimately, leveraging PTFE gaskets is a critical step in building a pharmaceutical manufacturing process that is safe, compliant, and consistently effective.
Summary Table:
| Key Property | Benefit in Pharma Manufacturing |
|---|---|
| Chemical Inertness | Prevents reactions with sensitive APIs and harsh cleaning agents. |
| Non-Porous Surface | Resists microbial growth and biofilm formation for sterility. |
| FDA Compliance | Meets stringent regulatory standards for safety and non-toxicity. |
| Heat & Chemical Resistance | Withstands high-temperature sterilization and aggressive cleanings. |
| Durability | Reduces maintenance costs and equipment downtime. |
Ensure Uncompromised Purity and Compliance with KINTEK PTFE Components
Is your pharmaceutical manufacturing process protected by the highest standard of sealing integrity? The right PTFE component is critical for safeguarding product purity, ensuring regulatory compliance, and maximizing operational uptime.
KINTEK manufactures precision PTFE components—including seals, gaskets, liners, and custom labware—specifically for demanding environments like yours. We serve the semiconductor, medical, laboratory, and industrial sectors, prioritizing precision in every part.
Partner with KINTEK to benefit from:
- Guaranteed Purity: Our chemically inert PTFE prevents contamination of sensitive Active Pharmaceutical Ingredients (APIs).
- Regulatory Confidence: Components designed to meet FDA and other stringent hygiene standards.
- Unmatched Durability: Withstand extreme process conditions, reducing maintenance and downtime.
- Custom Fabrication: From initial prototypes to high-volume production runs, we deliver solutions tailored to your exact equipment and process needs.
Let's discuss how our PTFE expertise can enhance your process. Contact KINTEK today for a consultation and quote.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
- Custom PTFE Teflon Balls for Advanced Industrial Applications
People Also Ask
- Can PTFE machined parts be customized according to specific requirements? Achieve Precision for Demanding Applications
- What are the main components of a PTFE sliding pad? A Simple Two-Part System for Low-Friction Movement
- In what applications are PTFE reducing flanges versatile? Solve Critical Connections in Demanding Industries
- What are the thermal properties of PTFE balls? Unlock Extreme Temperature Performance
- What are the processing methods for PTFE? A Guide to Compression Molding and Machining
- How is PTFE utilized in the food and beverage industry? Ensuring Safety and Efficiency
- What are the key components of a PTFE lined butterfly valve? The Engineered System for Corrosive & High-Purity Media
- What is the expected service life of expanded PTFE gaskets? Maximize Sealing Longevity in Harsh Environments



















