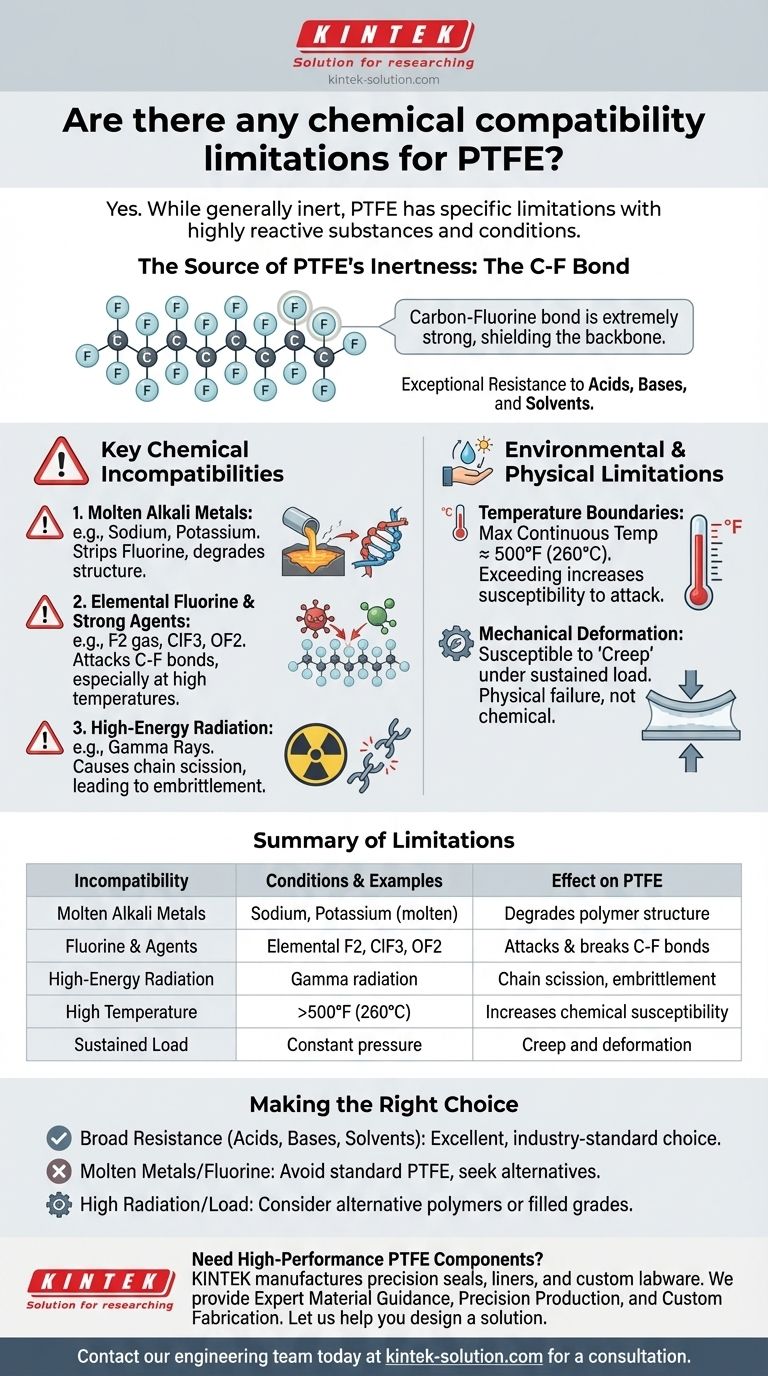
In short, yes. While Polytetrafluoroethylene (PTFE) is renowned for being one of the most chemically inert polymers available, it is not completely immune to attack. Its limitations are very specific, primarily involving highly reactive alkali metals in their molten state, elemental fluorine, and certain potent fluorinating agents, especially at elevated temperatures.
The exceptional chemical resistance of PTFE stems from its extremely strong carbon-fluorine bonds. Consequently, only a select few of the most aggressive and reactive chemical agents under specific conditions possess enough energy to break these bonds and degrade the material.

The Source of PTFE's Inertness
The Carbon-Fluorine Bond
PTFE's structure is a long chain of carbon atoms, each completely sheathed by fluorine atoms.
The bond between carbon and fluorine is one of the strongest known single bonds in organic chemistry. This molecular structure effectively shields the carbon backbone from chemical attack, making PTFE non-reactive to the vast majority of chemicals, including aggressive acids, bases, and solvents.
Key Chemical Incompatibilities
While its resistance is broad, there are well-defined exceptions where PTFE will fail. These situations typically involve chemicals that are reactive enough to disrupt the C-F bond.
Molten Alkali Metals
Alkali metals like sodium or potassium, when in their molten state, are powerful reducing agents. They are reactive enough to strip fluorine atoms from the PTFE polymer, causing a breakdown of the material's structure.
Elemental Fluorine and Strong Fluorinating Agents
It is a chemical reality that a substance can be attacked by its own constituent elements under the right conditions.
Gaseous or liquid fluorine, particularly when turbulent or under pressure, can attack PTFE. Similarly, highly reactive fluorinating compounds like chlorine trifluoride (ClF3) and oxygen difluoride (OF2) will degrade the polymer, especially at elevated temperatures.
Environmental and Physical Limitations
A complete technical evaluation must also consider factors beyond direct chemical compatibility that can lead to material failure.
High-Energy Radiation
PTFE does not have good resistance to high-energy radiation, such as gamma rays. This type of radiation can cause chain scission, which means the long polymer molecules are broken down into smaller pieces, leading to a rapid loss of mechanical properties and embrittlement.
Temperature and Pressure Boundaries
The maximum continuous operating temperature for PTFE is approximately 500°F (260°C). Approaching or exceeding this temperature can make the material more susceptible to attack by the aggressive chemicals previously mentioned.
Mechanical Deformation
It is critical to note that PTFE is a relatively soft material. It is susceptible to "creep" or deformation, especially under sustained load pressure. This is a physical limitation, not a chemical one, but it is a frequent cause of failure in poorly designed applications.
Making the Right Choice for Your Application
- If your primary focus is broad chemical resistance to acids, bases, solvents, or oils: PTFE is an excellent, industry-standard choice that will perform reliably within its temperature limits.
- If your application involves molten alkali metals, fluorine gas, or powerful fluorinating agents: You must avoid standard PTFE and seek alternative materials specifically designed for these extreme environments.
- If your application involves high radiation or significant, sustained mechanical load: You should consider alternative polymers or filled grades of PTFE that are engineered to improve radiation resistance and mechanical strength.
Understanding these specific limitations is the key to successfully leveraging PTFE's otherwise outstanding chemical inertness.
Summary Table:
| Incompatibility | Conditions & Examples | Effect on PTFE |
|---|---|---|
| Molten Alkali Metals | Sodium, Potassium (molten state) | Degrades polymer structure by stripping fluorine atoms |
| Fluorine & Fluorinating Agents | Elemental fluorine, ClF3, OF2 (esp. at high temp) | Attacks and breaks down the C-F bonds |
| High-Energy Radiation | Gamma radiation | Causes chain scission, leading to embrittlement |
| High Temperature | Exceeding 500°F (260°C) | Increases susceptibility to chemical attack |
| Sustained Mechanical Load | Constant pressure | Can lead to creep and deformation (physical failure) |
Need High-Performance PTFE Components for Demanding Applications?
PTFE's limitations are specific, but managing them is our specialty. At KINTEK, we manufacture precision PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors. We understand the critical balance between chemical resistance and mechanical performance.
We provide:
- Expert Material Guidance: Helping you select the right PTFE grade or alternative for your specific chemical and physical environment.
- Precision Production: Ensuring your components meet exact specifications for reliability.
- Custom Fabrication: From initial prototypes to high-volume production runs.
Let us help you design a solution that leverages PTFE's strengths while mitigating its risks.
Contact our engineering team today for a consultation to discuss your application requirements.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
- Custom PTFE Bottles for Diverse Industrial Applications
People Also Ask
- Is Teflon hard or soft compared to other engineering plastics? A Guide to Its Unique Properties
- Why is PTFE suitable for electrical applications? Discover Its Superior Insulating Properties
- What is PTFE material and what are its key properties? A Guide to the Ultimate High-Performance Polymer
- What are the key characteristics that make Teflon useful in industrial applications? Solve Tough Corrosion, Friction, and Temperature Challenges
- What are the main advantages of using NBR seat butterfly valves? A Cost-Effective Choice for Oil & Fuel Systems
- What gives PTFE its UV resistance? The Science of Inherent Molecular Stability
- What is the chemical composition of PTFE? Unlocking the Power of Carbon-Fluorine Bonds
- What are the advantages of PTFE in terms of shelf life and service intervals? Maximize Reliability and Minimize Downtime



















