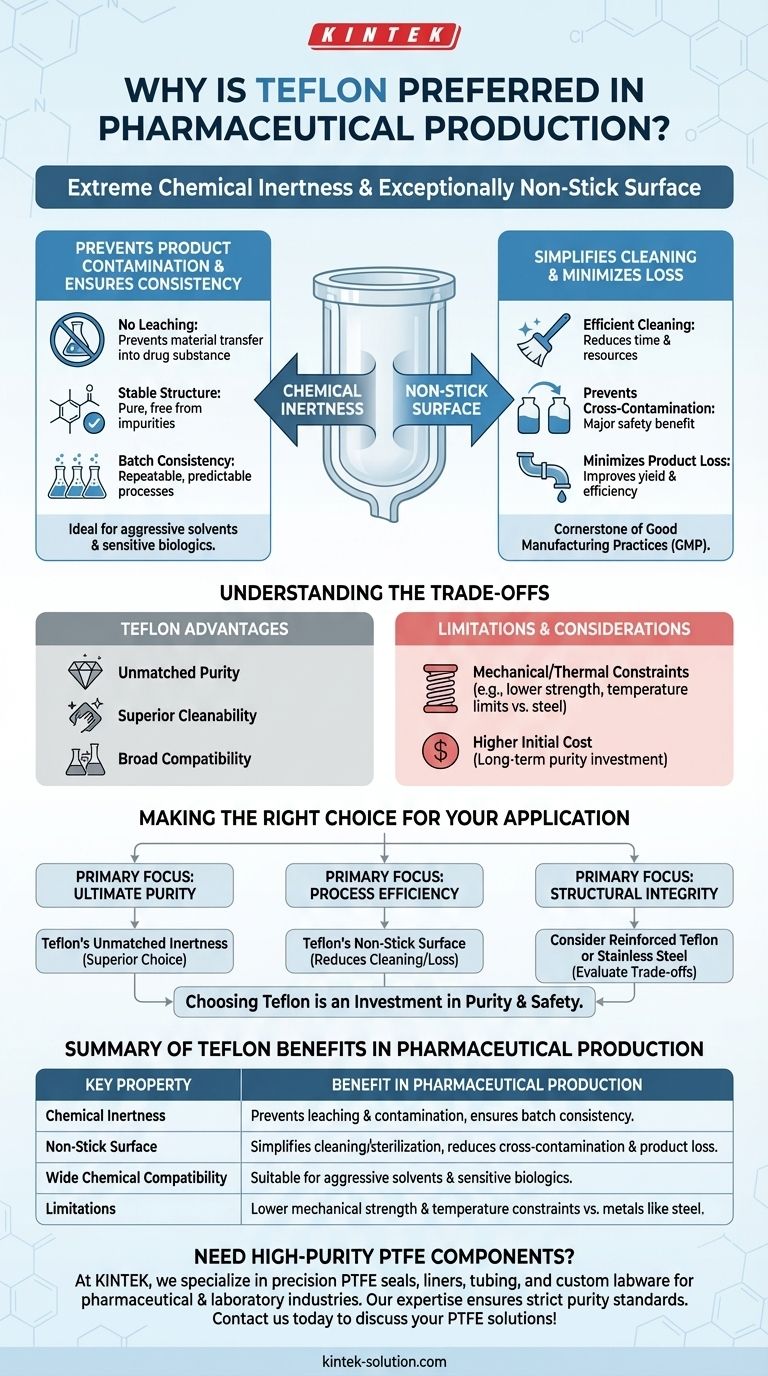
Teflon is the preferred material in pharmaceutical production for two fundamental reasons: its extreme chemical inertness and its exceptionally non-stick surface. These properties ensure that the material does not react with or contaminate the drug product, while also making equipment easier to clean and sterilize, which is critical for safety and purity.
The core challenge in pharmaceutical manufacturing is preventing product contamination and ensuring process consistency. Teflon directly solves this by providing a completely non-reactive and easily cleanable contact surface, safeguarding both the product's integrity and the patient's safety.

The Critical Role of Material Inertness
In pharmaceutical production, any interaction between the equipment and the product can have severe consequences. Teflon, a brand name for Polytetrafluoroethylene (PTFE), is valued because it is one of the most non-reactive materials known.
Preventing Product Contamination
Teflon’s stable chemical structure prevents it from leaching materials into the drug substance. This ensures the final product remains pure and free from unintended impurities that could alter its efficacy or cause harm.
This inertness applies across a wide range of chemicals, from aggressive solvents to sensitive biological compounds.
Ensuring Batch Consistency
Because Teflon does not participate in chemical reactions, its use in vessels, tubing, and seals guarantees that the manufacturing process is repeatable and predictable. The material will not act as an unintended catalyst or reactant, ensuring every batch meets the exact same specifications.
The Advantage of a Non-Stick Surface
Teflon's famous "non-stick" quality is just as important as its chemical stability in a pharmaceutical context. This property stems from its very low coefficient of friction.
Simplifying Cleaning and Sterilization
The non-stick surface prevents drug products and contaminants from adhering to equipment. This makes the cleaning and validation process vastly more efficient and reliable, which is a cornerstone of Good Manufacturing Practices (GMP).
Easier cleaning directly reduces the risk of cross-contamination between different product batches, a major regulatory and safety concern.
Minimizing Product Loss
In manufacturing, yield is critical. Teflon's slick surface ensures that the maximum amount of product is transferred through tubing and out of vessels, minimizing material loss and improving overall process efficiency.
Understanding the Trade-offs
While Teflon is an exceptional material, it is not a universal solution. A clear-eyed assessment requires acknowledging its limitations.
Mechanical and Thermal Constraints
Compared to materials like stainless steel, Teflon is softer and has lower mechanical strength. It can be susceptible to "creep" under sustained pressure and has more restrictive temperature limitations, which must be considered in process design.
Higher Initial Cost
Pharmaceutical-grade Teflon components are generally more expensive than their counterparts made from other polymers or even some metals. This cost must be weighed against the long-term benefits of enhanced purity, safety, and reduced cleaning efforts.
Making the Right Choice for Your Application
Selecting the correct material is a foundational decision in pharmaceutical process design. The choice hinges on balancing chemical compatibility with the physical demands of the operation.
- If your primary focus is ultimate purity and preventing product interaction: Teflon's unmatched chemical inertness makes it the superior choice, especially for sensitive biologics or processes involving aggressive chemicals.
- If your primary focus is process efficiency and cleanability: Teflon's non-stick surface minimizes product loss and significantly reduces the time and resources required for cleaning and sterilization between batches.
- If your primary focus is structural integrity under high pressure: You may need to consider reinforced Teflon components or stainless steel, carefully evaluating the trade-off between inertness and mechanical performance.
Ultimately, choosing a material like Teflon is a direct investment in the purity and safety of the final pharmaceutical product.
Summary Table:
| Key Property | Benefit in Pharmaceutical Production |
|---|---|
| Chemical Inertness | Prevents leaching and product contamination, ensuring batch consistency. |
| Non-Stick Surface | Simplifies cleaning/sterilization, reduces cross-contamination and product loss. |
| Wide Chemical Compatibility | Suitable for aggressive solvents and sensitive biologics. |
| Limitations | Lower mechanical strength and temperature constraints vs. metals like steel. |
Need high-purity PTFE components for your pharmaceutical application?
At KINTEK, we specialize in manufacturing precision PTFE seals, liners, tubing, and custom labware for the pharmaceutical, semiconductor, medical, and laboratory industries. Our expertise in custom fabrication—from prototypes to high-volume orders—ensures you get components that meet strict purity and safety standards.
Contact us today to discuss how our PTFE solutions can enhance your drug production process!
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- What advantages do PTFE syringe filters offer in laboratory settings? Unmatched Chemical Resistance & Purity
- What types of products are related to headspace septa? Essential Components for Leak-Proof Analysis
- What are the characteristics of narrow mouth PTFE laboratory bottles? Superior Chemical Resistance & Purity
- What steps are involved in selecting the proper PTFE filter? A 4-Step Guide to Optimal Filtration
- How does the manufacturer's reputation and quality assurance impact the choice of a PTFE-coated septum? Ensure Data Integrity
- Why are PTFE lined vials considered durable? Superior Chemical & Thermal Resistance for Reliable Performance
- How do PTFE/silicone septa maintain sample integrity in HPLC autosampler vials? Ensuring Accurate and Reliable Results
- What are the primary uses of PTFE silicone septa? Ensure Sample Integrity in GC/LC Analysis



















