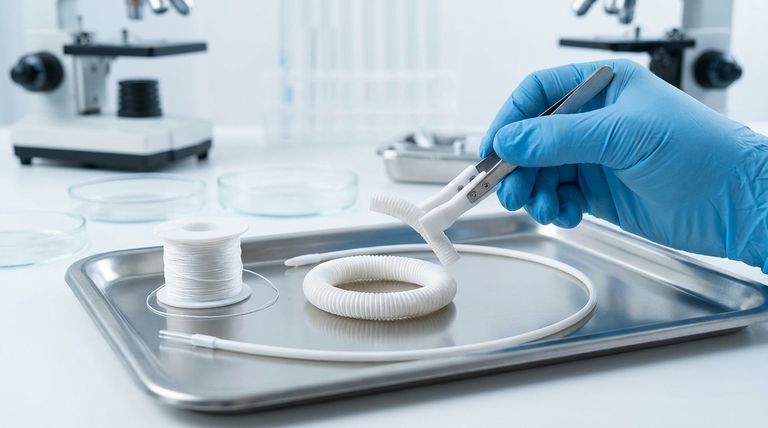
At its core, PTFE is approved for medical implants because it is exceptionally non-reactive. This property, known as being chemically and biologically inert, means the material can reside within the human body without triggering a significant immune response or degrading from contact with bodily fluids. The body effectively ignores its presence, making it a reliable choice for long-term implantation.
The value of PTFE in medicine stems not from what it does, but from what it doesn't do. Its extreme chemical stability allows it to exist within the body as a neutral, invisible barrier, preventing the adverse biological reactions that plague less stable materials.
The Foundation of PTFE's Biocompatibility
To understand why PTFE (Polytetrafluoroethylene) is so well-suited for medical use, we must look at its unique molecular structure. Its properties are not accidental; they are a direct result of its powerful chemical bonds.
The Unbreakable Carbon-Fluorine Bond
PTFE is a polymer made of a long chain of carbon atoms, where each carbon is completely shielded by fluorine atoms.
The bond between carbon and fluorine is one of the strongest single bonds in organic chemistry. This creates a tightly packed, protective sheath of fluorine atoms around the carbon backbone.
This molecular armor is the primary reason for PTFE's remarkable stability. It acts as a fortress, preventing other chemicals from getting close enough to react with the polymer chain.
Chemical Inertness and Resistance
Because of its strong C-F bonds, PTFE is resistant to virtually all chemicals, including the aggressive acids, enzymes, and fluids found within the human body.
Where other materials might corrode or break down over time, PTFE remains unchanged. This ensures the implant maintains its structural integrity and does not leach harmful substances into the patient's system.
Hydrophobicity and Low Surface Energy
PTFE is extremely hydrophobic, meaning it actively repels water and water-based substances like blood.
This property is critical because the first step in a foreign body reaction is often the adhesion of proteins and cells to the implant's surface. PTFE’s low surface energy makes it "non-stick," preventing this initial step and dramatically reducing the risk of blood clots (thrombosis) and immune rejection.
Practical Applications in Medical Implants
PTFE's unique combination of properties makes it ideal for specific medical applications where minimizing biological interaction is the primary goal.
Vascular Grafts
PTFE is frequently used to create artificial blood vessels, particularly for bypass surgery. Its smooth, non-stick surface helps prevent blood from clotting as it flows through the graft.
Surgical Sutures and Hernia Repair
The material's low coefficient of friction allows PTFE sutures to pass through delicate tissues with minimal damage. In mesh form, it provides a stable, non-reactive scaffold for hernia repair.
Coatings for Medical Devices
Thin coatings of PTFE are applied to devices like guidewires and catheters. This reduces friction, making the instruments easier to insert and maneuver within the body, which minimizes trauma to tissues.
Understanding the Limitations and Trade-offs
No material is perfect, and PTFE's strengths in one area create weaknesses in others. Its suitability is highly dependent on the specific application.
Poor Mechanical Wear Resistance
PTFE is a relatively soft material. It is not suitable for high-load, high-friction applications like artificial joints (e.g., hip or knee replacements).
Under significant mechanical stress, it can shed microscopic particles. While the material itself is inert, an accumulation of these particles can trigger a chronic inflammatory response from the body.
Difficulty in Sterilization
While chemically robust, PTFE can be sensitive to certain sterilization methods, particularly high-temperature steam or radiation, which can degrade its mechanical properties if not carefully controlled.
Lack of Tissue Integration
The same non-stick property that prevents blood clots also prevents natural tissue from growing onto and integrating with the implant. In applications where anchoring the device to the body is desired, this is a significant disadvantage, and other materials like titanium are often preferred.
Key Considerations for Material Selection
Your choice of material must be guided by the specific demands of the medical device and its intended biological environment.
- If your primary focus is a stable, non-reactive barrier: PTFE is an exceptional choice for applications like vascular grafts or coatings where you need to minimize all biological interaction.
- If your application involves high mechanical stress or requires tissue integration: You must consider alternative materials or composites, as PTFE's poor wear resistance and non-stick surface are critical limitations.
Understanding these fundamental properties allows you to leverage PTFE's unique strengths while respecting its critical limitations.
Summary Table:
| Key Property | Benefit for Medical Implants |
|---|---|
| Chemical Inertness | Resists bodily fluids, preventing degradation and adverse reactions. |
| Low Surface Energy | Repels proteins and cells, reducing risk of clotting and rejection. |
| Hydrophobicity | Prevents water absorption, maintaining structural integrity. |
| Smooth Surface | Minimizes tissue trauma, ideal for grafts and catheters. |
Leverage PTFE's Biocompatibility for Your Medical Device
At KINTEK, we specialize in the precision manufacturing of high-purity PTFE components—from seals and liners to custom labware—for the medical, semiconductor, and laboratory industries. Our expertise ensures your implants and devices meet the highest standards of biocompatibility and performance.
Whether you need prototypes or high-volume production, partner with us to create reliable, non-reactive PTFE solutions. Contact our team today to discuss your project requirements.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- What design considerations are important for custom PTFE parts? Design for Performance & Reliability
- What industrial benefits do PTFE-machined parts offer? Achieve Peak Performance in Demanding Applications
- What fabrication services are available for PTFE? Shearing, Stamping, Laser Cutting, Molding & Machining
- What challenges arise when machining PTFE (Teflon)? Overcome Softness, Heat, and Instability
- What factors should be considered when choosing between Nylon and PTFE? Select the Right Material for Your Application



















