At its core, chemical resistance is crucial in chromatography vials to ensure the vial material does not chemically interact with the sample, solvent, or standard it contains. This inertness is fundamental to preserving the sample's original chemical composition from the moment of collection to the point of injection, thereby guaranteeing the integrity and accuracy of the analytical results.
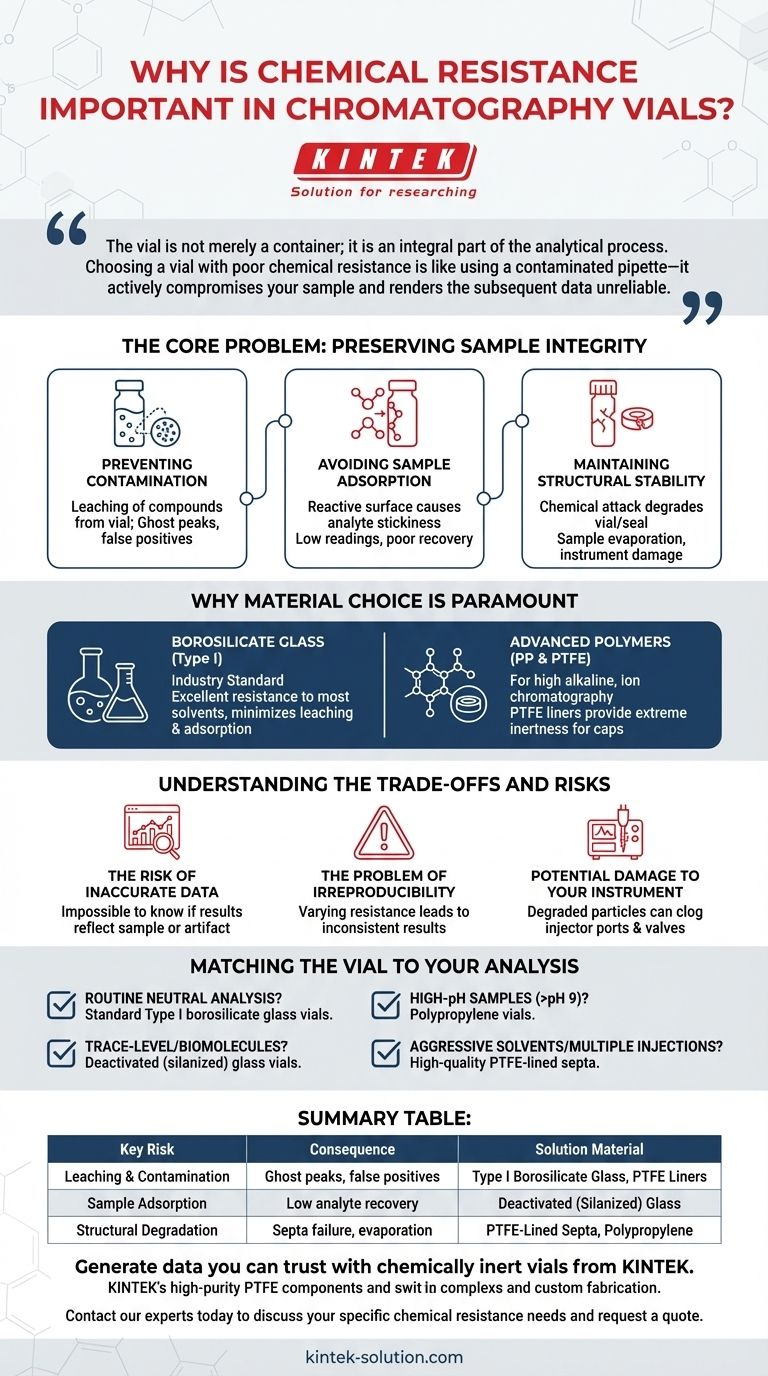
The vial is not merely a container; it is an integral part of the analytical process. Choosing a vial with poor chemical resistance is like using a contaminated pipette—it actively compromises your sample and renders the subsequent data unreliable.

The Core Problem: Preserving Sample Integrity
A vial's primary job is to be an invisible container. Any interaction, no matter how small, between the vial and the sample introduces a variable that can corrupt your analysis.
Preventing Contamination
The most significant risk of poor chemical resistance is the leaching of compounds from the vial material into your sample. Aggressive solvents, acids, or bases can break down the vial's glass or polymer structure, releasing extractables and leachables.
These contaminants can appear as ghost peaks in your chromatogram, mask peaks of interest, or artificially inflate the quantity of a target analyte, leading to false positives and inaccurate quantification.
Avoiding Sample Adsorption
The reverse problem is also common. A reactive vial surface can cause active analytes to adsorb, or "stick," to the vial walls.
This is particularly problematic in trace analysis, where even a small amount of analyte loss can dramatically alter the calculated concentration. The result is artificially low readings and poor recovery, leading to inaccurate conclusions.
Maintaining Structural Stability
Chemical attack doesn't just add or remove things from your sample; it can also degrade the physical structure of the vial or its seal.
A solvent may cause a plastic vial to swell or become brittle. More commonly, it can degrade the cap's septum, compromising the seal and allowing sample evaporation or contamination from the outside air.
Why Material Choice is Paramount
The vast majority of issues related to chemical resistance can be solved by selecting the appropriate material for the vial and the septa.
Borosilicate Glass: The Industry Standard
For most applications, Type I borosilicate glass is the material of choice. It offers excellent resistance to most common solvents and chemicals, providing a highly inert surface that minimizes the risk of both leaching and adsorption.
Advanced Polymers and Liners
In situations involving highly alkaline solutions (which can etch glass) or for specific applications like ion chromatography, vials made from polymers like polypropylene (PP) are used.
Similarly, the material of the cap's septum is critical. Septa are often lined with Polytetrafluoroethylene (PTFE), a polymer renowned for its extreme chemical inertness. As noted in process industries, PTFE can withstand nearly all aggressive acids, bases, and solvents without degrading.
Understanding the Trade-offs and Risks
Ignoring vial chemistry is not a shortcut; it's a direct path to flawed data and wasted resources.
The Risk of Inaccurate Data
The most immediate consequence is unreliable data. Whether through contamination or analyte loss, a non-resistant vial makes it impossible to know if your results reflect the true nature of your sample or an artifact of your container.
The Problem of Irreproducibility
If vials from different batches or manufacturers have varying levels of chemical resistance, your results will be inconsistent. This lack of reproducibility undermines the validity of any scientific study or quality control process.
Potential Damage to Your Instrument
Degraded particles from a vial or, more commonly, a septum can be drawn into the autosampler needle. This can lead to clogged injector ports, damaged valves, and contaminated columns, resulting in instrument downtime and expensive repairs.
Matching the Vial to Your Analysis
Choosing the right vial is a proactive measure to ensure data quality. Your decision should be based on the specific chemistry of your analysis.
- If your primary focus is routine analysis with neutral pH and common solvents: Standard Type I borosilicate glass vials provide a reliable and cost-effective solution.
- If your primary focus is trace-level analysis or working with sensitive biomolecules: Consider deactivated (silanized) glass vials to further minimize the risk of surface adsorption.
- If your primary focus is working with high-pH samples (>pH 9) or hydrofluoric acid: Polypropylene vials are often a safer choice to prevent the etching of glass.
- If your primary focus is using aggressive solvents or performing multiple injections: Ensure your vial caps have high-quality, chemically inert PTFE-lined septa to prevent contamination and ensure a consistent seal.
Ultimately, selecting a chemically resistant vial is the first and most critical step in generating data you can trust.
Summary Table:
| Key Risk | Consequence | Solution Material |
|---|---|---|
| Leaching & Contamination | Ghost peaks, false positives | Type I Borosilicate Glass, PTFE Liners |
| Sample Adsorption | Low analyte recovery, inaccurate quantification | Deactivated (Silanized) Glass |
| Structural Degradation | Septa failure, sample evaporation | PTFE-Lined Septa, Polypropylene |
Generate data you can trust with chemically inert vials from KINTEK.
Your chromatography is only as reliable as your vial. KINTEK specializes in manufacturing high-purity PTFE components—including seals, liners, and labware—and supplying compatible vials for the most demanding applications in semiconductor, medical, laboratory, and industrial settings.
We prioritize precision production and offer custom fabrication from prototypes to high-volume orders to meet your exact specifications. Ensure your sample integrity from collection to injection.
Contact our experts today to discuss your specific chemical resistance needs and request a quote.
Visual Guide
Related Products
- Customizable PTFE Seals Filter Holders for Versatile Applications
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- PTFE Deep Evaporating Dishes Customizable Laboratory and Industrial Solutions
- Custom PTFE Shallow Evaporating Dishes for Diverse Applications
- Customizable PTFE Crucibles for Laboratory and Industrial Applications
People Also Ask
- What material is the PTFE bottle made from? Discover the Benefits of 100% Virgin PTFE
- What factors should be considered when choosing between PTFE lined and hard seal butterfly valves? Ensure Optimal Performance and Safety
- What are the key features of PTFE-lined bottle caps? Ensure Chemical Integrity and Purity for Your Samples
- How do PTFE properties benefit butterfly valve performance? Enhance Durability & Efficiency
- How does PTFE perform in chemically corrosive environments? Unmatched Chemical Immunity & Reliability






