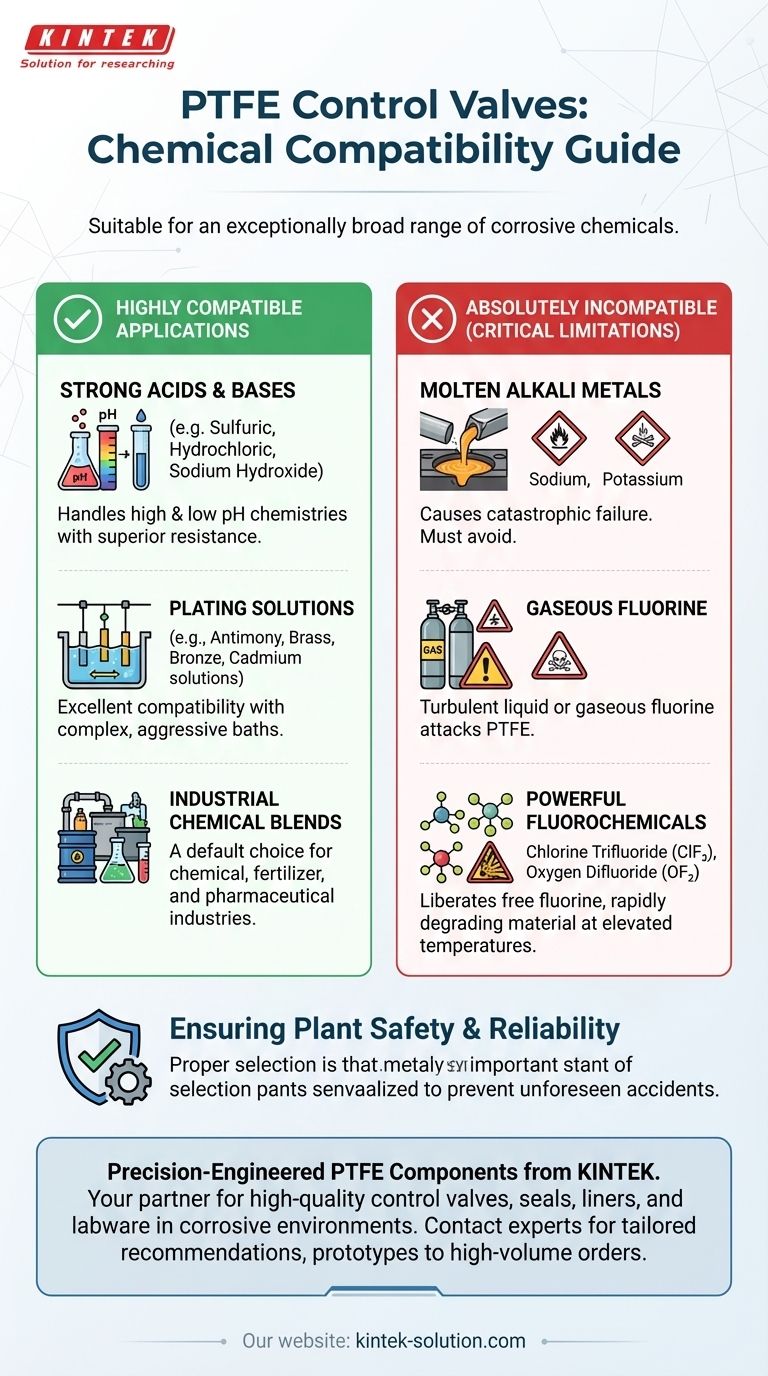
In short, PTFE control valves are suitable for an exceptionally broad range of chemicals, particularly those that are highly corrosive. They are a default choice for handling both high and low P.H. chemistries, including strong acids, bases, various chemical blends, and a wide array of industrial plating solutions.
PTFE's near-universal chemical inertness makes it one of the most reliable materials for control valves in corrosive environments. However, its value lies not just in what it resists, but in clearly understanding the few, specific chemicals that can cause it to fail catastrophically.

The Foundation of PTFE's Chemical Resistance
Polytetrafluoroethylene (PTFE) is a fluoropolymer with unique properties that make it a go-to material for demanding chemical applications. Its resilience is not accidental; it's a result of its molecular structure.
An Exceptionally Inert Material
The bonds between carbon and fluorine atoms in PTFE's structure are extremely strong and stable. This molecular stability is the reason PTFE is so non-reactive, refusing to engage with most chemicals it contacts.
Broad Industrial Application
Because of this inertness, PTFE control valves are specified across numerous sectors. They are a common sight in the chemical, fertilizer, and pharmaceutical industries, where material purity and resistance to corrosion are paramount.
Key Areas of Compatibility
PTFE's versatility allows it to be used in a variety of aggressive chemical services where lesser materials would quickly degrade.
Strong Acids and Bases
PTFE valves excel in managing fluids at both ends of the pH scale. They are particularly suitable for strong acids and caustic solutions that would rapidly corrode most metals and other polymers.
Specialized Plating Solutions
The material demonstrates excellent compatibility with many complex and often aggressive plating solutions used in metal finishing.
Examples include:
- Antimony and Arsenic plating baths
- Brass and Bronze plating solutions (Cu-Cd, Cu-Sn, Cu-Zn)
- Cadmium plating baths (both cyanide and fluoborate types)
Critical Limitations: When Not to Use PTFE
No material is universally perfect. Understanding PTFE's specific vulnerabilities is essential for ensuring plant safety and reliability. A failure in these applications is not gradual; it can be sudden and severe.
The Absolute Incompatibility List
PTFE is highly susceptible to attack from a very specific list of substances. You must avoid using PTFE control valves with:
- Molten alkali metals, such as sodium or potassium.
- Turbulent liquid or gaseous fluorine.
- Certain powerful fluorochemicals, such as chlorine trifluoride (ClF₃) or oxygen difluoride (OF₂).
The Danger of Elevated Temperatures
The risk associated with some of these incompatible chemicals increases dramatically at higher temperatures. Compounds like ClF₃ can liberate free fluorine, which aggressively attacks the PTFE polymer structure, leading to rapid material failure.
Improving Plant Safety and Reliability
Using PTFE correctly is a critical safety function. Selecting it for compatible services helps prevent unforeseen accidents caused by corrosion and material failure, directly improving the overall reliability and safety of the facility.
Making the Right Choice for Your Application
Your decision must be based on a clear understanding of your specific process chemistry and conditions.
- If your primary focus is handling strong acids, bases, or most industrial chemical blends: PTFE is an excellent and highly reliable choice known for its superior corrosion resistance.
- If your primary focus is a process involving molten alkali metals or specific fluorine-releasing compounds: You must specify an alternative material, as using PTFE presents a significant and unacceptable safety risk.
- If your primary focus is on high-temperature service: Always verify your specific chemical against PTFE's known compatibility data at your target operating temperature, as reactivity can change.
Ultimately, proper material selection by understanding both the strengths and the critical exceptions of PTFE is fundamental to designing a safe and efficient system.
Summary Table:
| Chemical Compatibility | Status | Key Examples |
|---|---|---|
| Highly Compatible | ✅ Excellent Resistance | Strong acids (e.g., Sulfuric, Hydrochloric), Strong bases (e.g., Sodium Hydroxide), Most industrial plating solutions |
| Absolutely Incompatible | ❌ High Risk of Failure | Molten alkali metals (Sodium, Potassium), Gaseous Fluorine, Chlorine Trifluoride (ClF₃) |
Ensure the safety and reliability of your chemical processes with precision-engineered PTFE components from KINTEK.
We specialize in manufacturing high-quality PTFE control valves, seals, liners, and labware for the semiconductor, medical, laboratory, and industrial sectors. Our expertise ensures your equipment can handle even the most corrosive environments.
Let us provide the right solution for your needs, from custom prototypes to high-volume orders.
Contact our experts today to discuss your specific chemical application and receive a tailored recommendation.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Customizable PTFE Seals Filter Holders for Versatile Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- What are some important physical property values for PTFE? Master Its Extreme Performance for Demanding Applications
- What is the temperature range that PTFE can withstand? From -200°C to +260°C for Demanding Applications
- When and by whom was PTFE discovered? A Tale of Accidental Innovation
- What is the hardness range of PTFE on the Shore D scale? Leveraging Its Softness for Superior Performance
- How is PTFE used in industrial processes? Maximize Safety and Efficiency



















