In short, very few substances can affect the incredibly strong carbon-fluorine bonds of Polytetrafluoroethylene (PTFE). This near-invulnerability is the material's defining characteristic. However, a specific set of highly reactive chemicals, often under extreme conditions like high temperatures or pressures, can successfully attack and degrade the polymer.
PTFE's legendary chemical inertness stems from the strength and stability of its carbon-fluorine bonds. Only the most aggressive chemical agents, such as molten alkali metals or specific fluorine compounds under heat and pressure, possess enough energy to break these bonds.

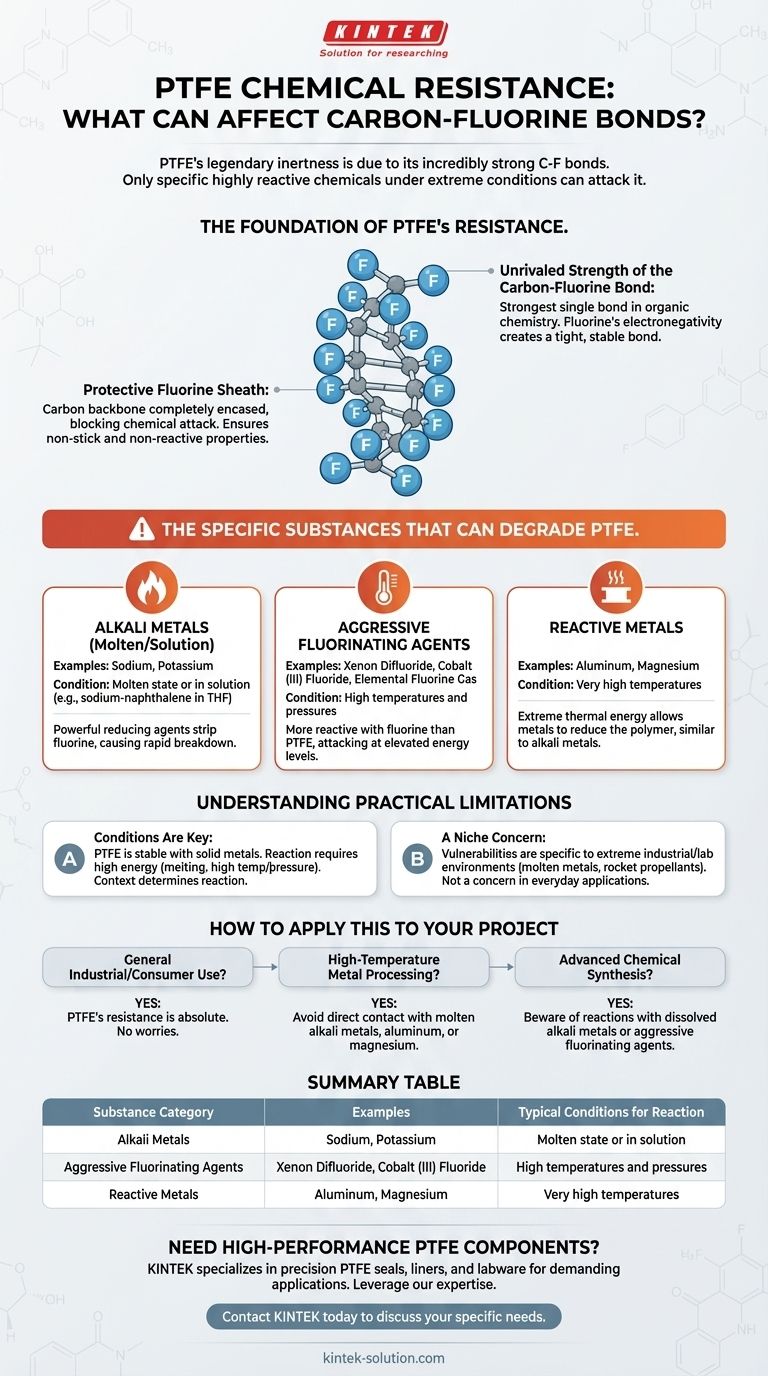
The Foundation of PTFE's Chemical Resistance
To understand what can break down PTFE, it's essential to first understand why it is so remarkably stable. The material's properties are a direct result of its molecular structure.
The Unrivaled Strength of the Carbon-Fluorine Bond
The bond between carbon and fluorine atoms is one of the strongest single bonds in organic chemistry.
Fluorine is the most electronegative element, meaning it pulls bonding electrons very tightly towards itself. This creates a short, strong, and highly stable bond that is difficult to break.
A Protective Fluorine Sheath
In PTFE, the carbon backbone is completely encased in a dense, helical sheath of fluorine atoms. This sheath physically protects the vulnerable carbon chain from potential chemical attack.
This structure leaves no easy point of entry for most chemicals to initiate a reaction, making the material non-stick and extremely non-reactive.
The Specific Substances That Can Degrade PTFE
While PTFE is resistant to virtually all common acids, bases, solvents, and oxidizers, a few specific categories of substances can overcome its defenses.
Alkali Metals (Molten or in Solution)
This is the most well-known exception to PTFE's chemical resistance. Molten alkali metals like sodium or potassium, or their solutions (e.g., sodium-naphthalene in THF), are powerful reducing agents.
These metals are reactive enough to strip fluorine atoms from the polymer backbone, causing a rapid and complete breakdown of the material's structure.
Aggressive Fluorinating Agents
Certain rare and highly reactive fluorinating compounds can attack PTFE, but typically only at elevated temperatures and pressures.
Examples include xenon difluoride and cobalt (III) fluoride. Essentially, these are substances even more reactive with fluorine than PTFE itself. Elemental fluorine gas at high temperatures also falls into this category.
Reactive Metals at High Temperatures
Specific metals, most notably aluminum and magnesium, can react with PTFE at very high temperatures.
The extreme thermal energy overcomes the bond's stability, allowing these metals to reduce the polymer in a manner similar to alkali metals, though typically under more extreme heat.
Understanding the Practical Limitations
It is critical to recognize that these vulnerabilities exist under very specific and uncommon conditions. For the vast majority of applications, they are not a practical concern.
Conditions Are as Important as the Chemical
PTFE is perfectly stable in contact with solid aluminum or sodium at room temperature. The reaction only occurs when sufficient energy is introduced, such as melting the metal.
The context of temperature, pressure, and physical state (molten, dissolved) is the deciding factor in whether a reaction will occur.
A Niche Concern, Not a Common Weakness
These chemical incompatibilities are primarily a concern in highly specialized industrial or laboratory environments.
Settings involving molten metal processing, rocket propellants, or advanced chemical synthesis are where these limitations must be considered. In everyday applications, these conditions are virtually never encountered.
How to Apply This to Your Project
Understanding these limits allows you to deploy PTFE with confidence, ensuring its legendary performance and reliability where it matters most.
- If your primary focus is general industrial or consumer use: PTFE's chemical resistance is effectively absolute, and you do not need to worry about these specific exceptions.
- If your primary focus is high-temperature metal processing: You must avoid using PTFE in direct contact with molten alkali metals, aluminum, or magnesium.
- If your primary focus is advanced chemical synthesis: Be aware that PTFE may not be suitable for reactions involving dissolved alkali metals or aggressive fluorinating agents.
Ultimately, knowing the specific, well-defined limits of an exceptionally robust material like PTFE is the key to using it effectively.
Summary Table:
| Substance Category | Examples | Typical Conditions for Reaction |
|---|---|---|
| Alkali Metals | Sodium, Potassium | Molten state or in solution (e.g., sodium-naphthalene) |
| Aggressive Fluorinating Agents | Xenon Difluoride, Cobalt (III) Fluoride | High temperatures and pressures |
| Reactive Metals | Aluminum, Magnesium | Very high temperatures |
Need High-Performance PTFE Components for Demanding Applications?
At KINTEK, we specialize in manufacturing precision PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors. Our expertise ensures your materials can withstand even the most challenging chemical environments.
Let us provide the reliable, custom-fabricated PTFE solutions your project requires, from prototypes to high-volume orders.
Contact KINTEK today to discuss your specific needs and leverage our material science expertise.
Visual Guide
Related Products
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Customizable PTFE Crucibles for Laboratory and Industrial Applications
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
People Also Ask
- What is Teflon and what is its chemical name? Unpacking the Science of PTFE
- What environmental resistances does PTFE offer? Unmatched Durability for Harsh Conditions
- What is PTFE commonly known as and what type of material is it? A Guide to High-Performance PTFE Properties
- What is PTFE and what class of plastics does it belong to? A Guide to High-Performance Fluoropolymers
- When was PTFE discovered and developed? The Accidental Invention That Changed Industries



















