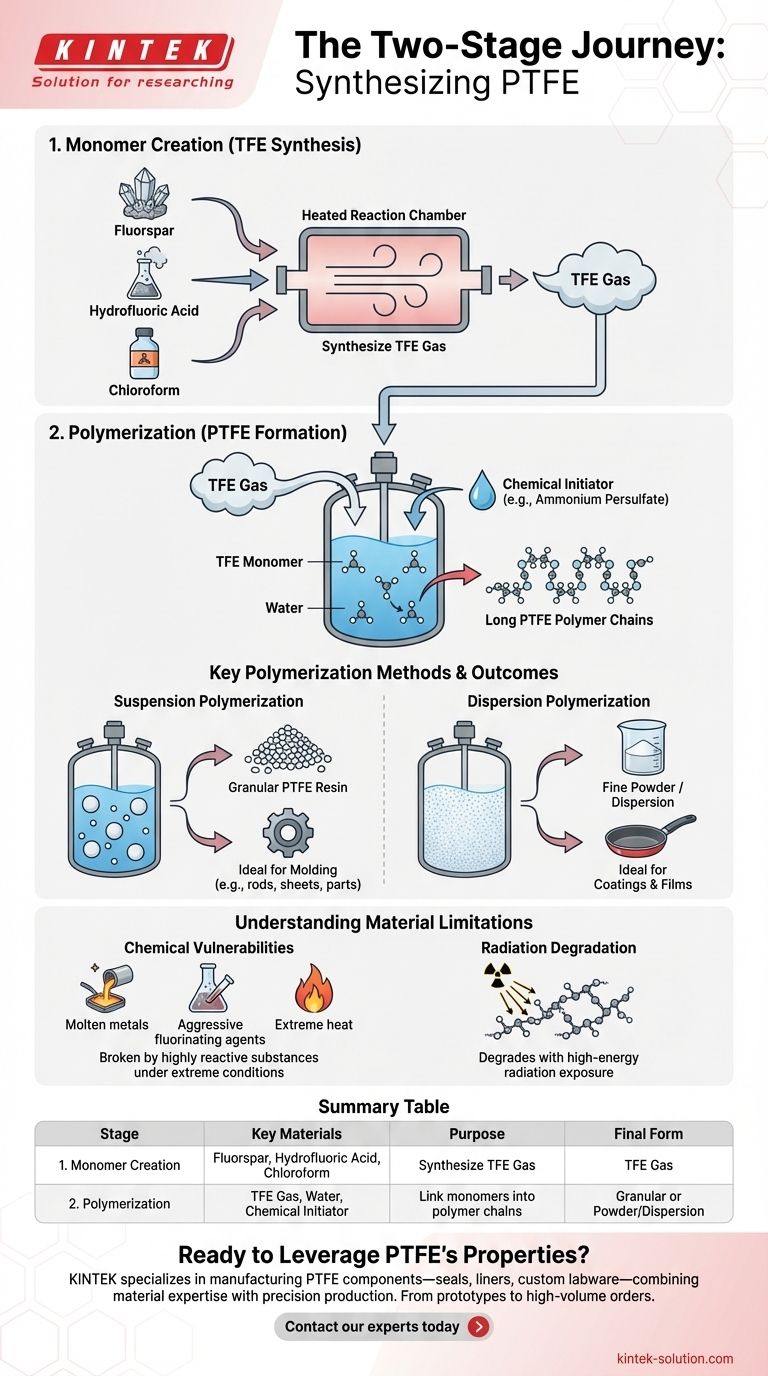
Synthesizing Polytetrafluoroethylene (PTFE), the polymer widely known by the trade name Teflon, is a multi-stage chemical process. It begins with three primary raw materials: fluorspar, hydrofluoric acid, and chloroform. These precursors are used to create an intermediate gas, which is then polymerized using water and a chemical initiator to form the final PTFE material.
The creation of PTFE is a two-step journey. First, basic chemical feedstocks are reacted to synthesize tetrafluoroethylene (TFE) gas. Second, this TFE gas is subjected to polymerization, where individual molecules are linked into the long, stable chains that give PTFE its unique properties.

The Two-Stage Synthesis Process
The industrial production of PTFE is not a single reaction but a sequence of distinct chemical transformations. Understanding these two stages is key to understanding the material itself.
Stage 1: Creating the Monomer (TFE)
The foundation of PTFE is its monomer, a gas called tetrafluoroethylene (TFE). This gas is synthesized first.
The core ingredients—fluorspar, hydrofluoric acid, and chloroform—are reacted inside a specialized, heated chamber. This chemical reaction re-arranges the molecules to produce TFE, the essential building block for the final polymer.
Stage 2: Polymerization into PTFE
Once TFE gas is created and purified, it undergoes polymerization. This is the process of linking many individual TFE molecules (monomers) into massive chains (the polymer).
This stage requires water, which typically serves as the medium for the reaction. Critically, a small amount of a chemical initiator, such as ammonium persulfate, is added. The initiator is the catalyst that kicks off the chain reaction, causing the TFE molecules to bond together into the stable structure of PTFE.
Key Polymerization Methods and Their Outcomes
The specific method used during the polymerization stage determines the physical form of the final PTFE product. The two primary methods result in materials suited for very different applications.
Suspension Polymerization
In this method, the polymerization reaction occurs with the TFE suspended in water.
The result is a granular PTFE resin, which looks like small grains or pellets. This form is ideal for molding processes, such as creating solid rods, sheets, or complex machined parts.
Dispersion Polymerization
This process creates a much finer product. The resulting PTFE consists of very small particles dispersed in water, forming a milky liquid.
This dispersion can be used directly for coatings or dried to produce a fine powder. This is the method used to create the non-stick coatings on cookware and other surface treatments.
Understanding the Material's Limitations
While renowned for its chemical inertness and low friction, PTFE is not invincible. Its synthesis process results in a molecular structure with specific vulnerabilities.
Chemical Vulnerabilities
PTFE’s powerful carbon-fluorine bonds can be broken by a few highly reactive substances, typically under extreme conditions.
These include molten alkali metals (like sodium), rare and aggressive fluorinating agents (like xenon difluoride and chlorine trifluoride), and certain metals at very high temperatures. For most practical purposes, however, it remains one of the most chemically resistant plastics available.
Degradation from Radiation
PTFE does not have good resistance to high-energy radiation. Exposure to gamma rays or electron beams can cause the long polymer chains to break, degrading the material's mechanical properties and making it brittle.
Making the Right Choice for Your Goal
The synthesis method directly dictates the material's form and ultimate use case.
- If your primary focus is creating solid, molded parts: You need granular PTFE produced via suspension polymerization, which is designed for compression molding and machining.
- If your primary focus is developing surface coatings or films: You require the fine powder or liquid dispersion created by dispersion polymerization, which is formulated for application and sintering.
Understanding the synthesis pathway is the first step to leveraging the unique properties of PTFE for any engineering challenge.
Summary Table:
| Stage | Key Materials | Purpose |
|---|---|---|
| 1. Monomer Creation | Fluorspar, Hydrofluoric Acid, Chloroform | Synthesize TFE gas, the building block of PTFE. |
| 2. Polymerization | TFE Gas, Water, Chemical Initiator | Link monomers into long polymer chains to form PTFE. |
| Final Form | Granular Resin (Suspension) or Fine Powder/Dispersion (Dispersion) | Determines the application method (molding vs. coating). |
Ready to leverage PTFE's properties for your project?
Understanding the synthesis of PTFE is the first step. The next is sourcing or fabricating the high-precision PTFE components your application demands.
KINTEK specializes in manufacturing PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors. We combine expertise in material science with precision production, offering custom fabrication from prototypes to high-volume orders.
Let us help you turn material knowledge into a high-performance solution.
Contact our experts today to discuss your specific requirements and get a quote.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- PTFE Chemical Solvent Sampling Spoon
People Also Ask
- How is Teflon utilized in the automotive industry? Enhance Vehicle Efficiency and Durability
- Why are fillers added to PTFE? Enhance Performance for Demanding Applications
- What are the environmental impacts of PTFE production? The Truth About PFAS & 'Forever Chemicals'
- What are the key physical and chemical properties of PTFE? Unlock Unmatched Chemical & Thermal Resistance
- What should be considered before specifying PTFE material for an application? Avoid Costly Design Failures
- Is PTFE safe for use in cooking equipment? Modern Non-Stick Cookware Safety Explained
- What are filled PTFE resins and how are they produced? A Guide to Enhanced Performance Materials
- What are the general properties of PTFE? Master Its Extreme Performance for Demanding Applications



















