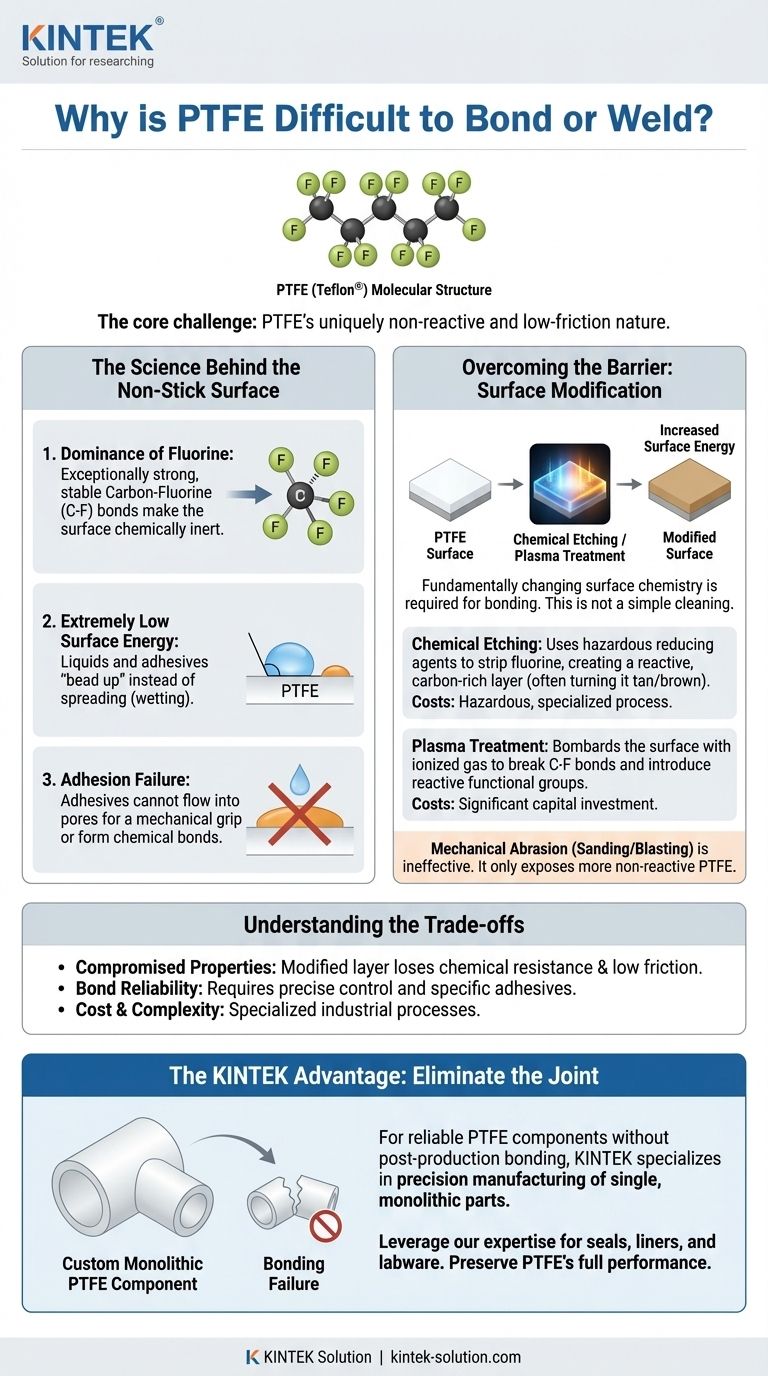
The fundamental challenge with PTFE is its uniquely non-reactive and low-friction nature. This material, commonly known by the brand name Teflon®, is difficult to adhere to or weld because its chemical structure creates one of the lowest surface energies of any known solid. Adhesives and other materials are physically repelled from the surface, preventing them from spreading out and forming a strong bond.
The core problem is not the adhesive, but the PTFE surface itself. To achieve any successful bond, the surface of the PTFE must be chemically altered through a process like etching, which fundamentally changes its properties to make it receptive to adhesion.
The Science Behind PTFE's Non-Stick Surface
To understand why PTFE resists bonding, we must look at its molecular structure. The material's properties are not accidental; they are a direct result of its specific chemical composition.
The Dominance of Fluorine
PTFE consists of a long chain of carbon atoms completely shielded by a sheath of fluorine atoms. The carbon-fluorine (C-F) bond is exceptionally strong and stable.
Fluorine atoms are highly electronegative, meaning they hold onto their electrons tightly and are not inclined to share them or react with other chemicals. This creates an electrically neutral, non-polar, and chemically inert surface.
Understanding Surface Energy
Think of surface energy as the "desire" of a surface to bond. High-energy surfaces are reactive and allow liquids, like adhesives, to spread out easily in a process called "wetting."
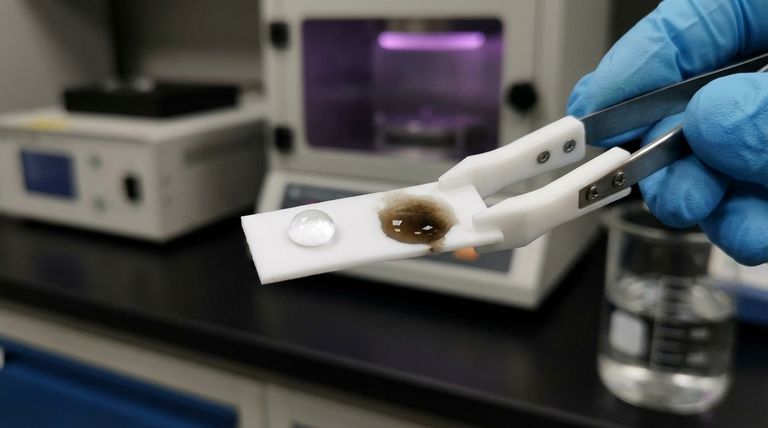
PTFE has an extremely low surface energy. Instead of wetting the surface, liquids bead up, minimizing their contact area. This is the same phenomenon you see when water beads on a freshly waxed car. For an adhesive to work, its surface tension must be lower than the surface energy of the material it is bonding to, which is nearly impossible with untreated PTFE.
The Impact on Adhesion and Welding
Because adhesives cannot wet the surface, they cannot flow into microscopic pores to create a mechanical grip, nor can they form the chemical bonds necessary for a durable connection.
While thermal welding is a different process, it is also complicated by PTFE's properties. The material has a high melting point and does not flow easily like other thermoplastics, making fusion welding a highly specialized and difficult task.
Overcoming the Adhesion Barrier: Surface Modification
You cannot successfully glue raw PTFE. The solution is to prepare the material by fundamentally changing its surface chemistry. This is not a simple cleaning or abrasion; it is a chemical transformation.
Chemical Etching
The most common industrial method is chemical etching. This involves exposing the PTFE surface to a potent reducing agent, typically a solution of sodium in liquid ammonia or a sodium naphthalene complex.
This process violently strips fluorine atoms from the polymer backbone. This leaves behind a carbon-rich, defluorinated layer that has a much higher surface energy. The visible result is the PTFE turning a tan or dark brown color. This "etched" surface is now receptive to adhesives like epoxies or cyanoacrylates.
Plasma Treatment
A more modern and controlled method is plasma treatment. The PTFE is placed in a vacuum chamber where a gas (like oxygen, hydrogen, or argon) is ionized to create a plasma.
This plasma bombards the surface, breaking the C-F bonds and introducing new, reactive functional groups. This increases the surface energy, making the PTFE bondable without the use of hazardous wet chemicals.
Mechanical Abrasion (And Why It's Insufficient)
Many attempt to sand or grit-blast PTFE to create a rougher profile for an adhesive to grip. While this slightly increases the surface area, it is largely ineffective.
The process merely exposes more of the same low-energy, non-reactive PTFE surface. Without a chemical change, a strong adhesive bond cannot be formed.
Understanding the Trade-offs
Modifying the surface of PTFE is a powerful technique, but it is essential to understand the compromises involved.
Cost and Process Complexity
Both chemical etching and plasma treatment are specialized industrial processes. The chemicals used in etching are highly hazardous and require expert handling and disposal. Plasma equipment is a significant capital investment. This makes proper PTFE bonding difficult and expensive for hobbyists or small-scale applications.
Compromised Surface Properties
The etched layer that allows for bonding no longer possesses PTFE's hallmark properties. The treated surface loses its extreme chemical resistance, low coefficient of friction, and dielectric strength. The bond is only as strong as this thin, modified layer.
Bond Reliability
Even with proper surface treatment, bonding PTFE requires careful selection of adhesives and precise process control. The bond is a common point of failure if the etching is inconsistent or the wrong adhesive is used for the application's thermal and mechanical demands.
Making the Right Choice for Your Goal
The correct approach depends entirely on your resources and final objective.
- If your primary focus is a small-scale repair or prototype: Your most practical option is to either purchase pre-etched PTFE film or tape from a specialty supplier or abandon adhesion and use mechanical fasteners (screws, rivets) instead.
- If you are designing for an industrial or manufacturing process: You must incorporate surface modification into your production. Partner with a company that specializes in chemical etching or plasma treatment of fluoropolymers.
- If your application requires the joint to maintain full chemical inertness: Adhesive bonding is not a suitable solution. You must investigate advanced thermal welding techniques or redesign the part to be monolithic and avoid a joint altogether.
Understanding PTFE's unique surface chemistry is the key to successfully engineering solutions that leverage its remarkable properties.
Summary Table:
| Challenge | Root Cause | Primary Solution |
|---|---|---|
| Adhesion Failure | Extremely low surface energy; adhesives cannot 'wet' the surface. | Chemical surface modification (etching or plasma). |
| Welding Difficulty | High melting point and poor flow characteristics. | Specialized thermal welding techniques. |
| Insufficient Methods | Abrasion only exposes more non-reactive PTFE. | Requires a chemical change to the surface layer. |
Need a reliable PTFE component that doesn't require post-production bonding?
At KINTEK, we specialize in the precision manufacturing of custom PTFE parts—from seals and liners to complex labware—for the semiconductor, medical, and industrial sectors. By fabricating your component as a single, monolithic piece, we eliminate the need for problematic bonding and preserve PTFE's full chemical inertness and performance.
Let us deliver a solution that meets your exact specifications, from prototype to high-volume production. Contact our experts today to discuss your project and leverage our expertise in high-performance polymers.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
People Also Ask
- What is PTFE and its basic chemical structure? The Key to Its Legendary Performance
- What are the key chemical properties of PTFE that make it useful for cookware? Unlock Superior Non-Stick Performance
- What is PTFE and what are its main properties? Discover the Ultimate High-Performance Polymer
- What is PTFE and when was it discovered? The Accidental Invention That Changed Industries
- How is PTFE laminated fabric manufactured? A Guide to High-Performance Material Engineering
- What were the unexpected properties of the newly discovered PTFE? Unveiling the Game-Changing Material
- How does PTFE benefit aerospace applications? Achieve Superior Performance in Extreme Environments
- What are the key features of PTFE laminated membrane filters? Hydrophobic, Chemically Inert, and Durable



















