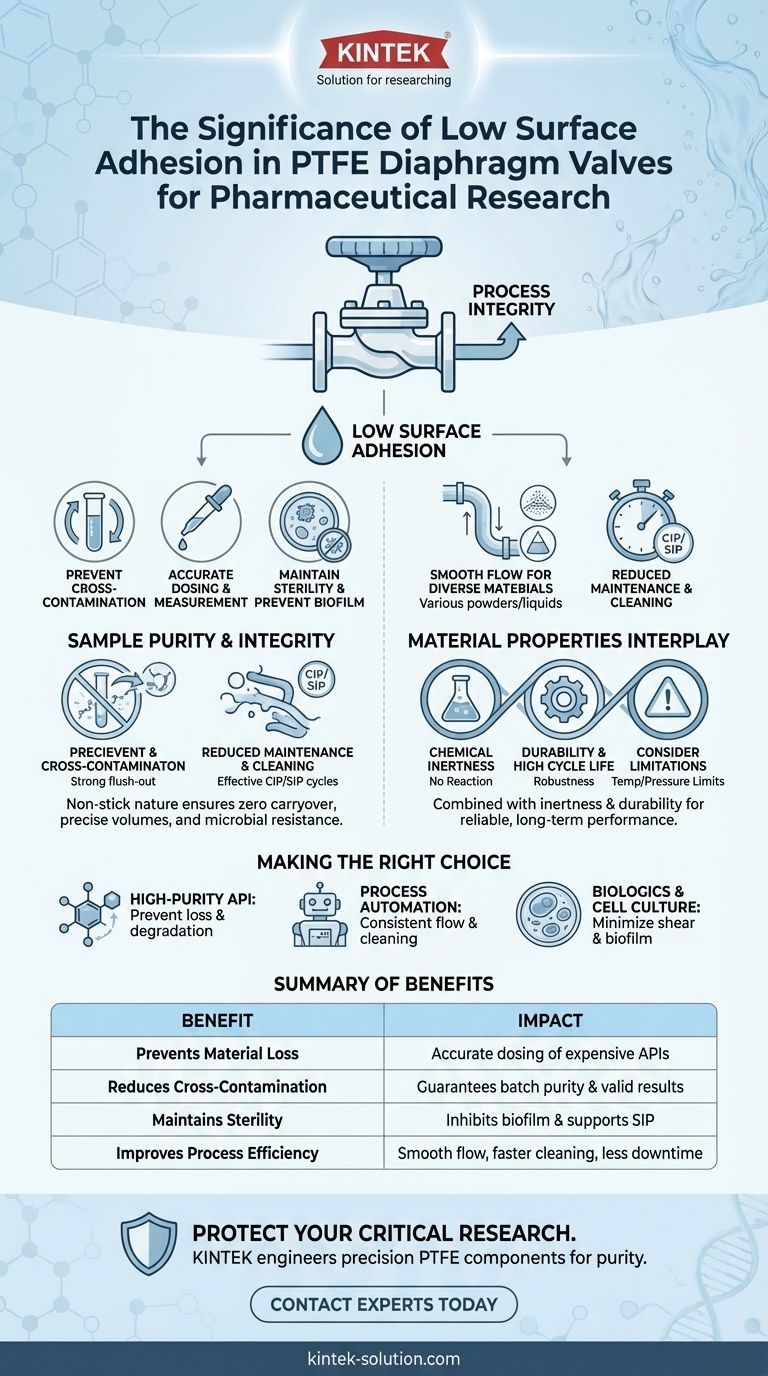
In pharmaceutical research, process integrity is non-negotiable. The low surface adhesion of Polytetrafluoroethylene (PTFE) is significant because it prevents materials—whether powders, liquids, or biologics—from sticking to the valve's internal surfaces. This single property directly minimizes material loss, reduces the risk of cross-contamination between batches, and ensures consistent, predictable flow control, which are all critical for valid and repeatable experimental outcomes.
The core issue isn't just about preventing stickiness. It's about ensuring that the substance entering the valve is the exact same substance—in composition and quantity—that exits. Low surface adhesion is a fundamental enabler of sample integrity and process reliability.

The Critical Role of Adhesion in Sample Purity
In research, the slightest alteration of a sample can invalidate results. The non-stick nature of PTFE provides a crucial layer of defense for the product moving through a fluid path.
Preventing Cross-Contamination
When material from a previous batch clings to a valve surface, it can easily leach into the next batch. This creates an immediate risk of cross-contamination.
PTFE’s low surface adhesion ensures that components are flushed out completely during cleaning cycles. This makes for a truly clean surface, ready for the next high-purity substance without carrying over contaminants.
Ensuring Accurate Dosing and Measurement
If a portion of a valuable Active Pharmaceutical Ingredient (API) or compound adheres to the valve, the final delivered dose will be inaccurate. This loss of material can compromise experimental results.
Because PTFE minimizes material cling, it ensures that the volume or mass you intend to transfer is what is actually delivered. This is essential for the precision required in formulation and analytical studies.
Maintaining Sterility and Preventing Biofilm
Sticky surfaces are ideal breeding grounds for microbial growth and biofilm formation, a major threat in sterile processes.
The smooth, non-stick surface of a PTFE diaphragm is much more difficult for microbes to colonize. It also makes sterilization processes, such as Sterilization-in-Place (SIP), more effective and reliable.
How Low Adhesion Drives Process Efficiency
Beyond purity, the physical properties of PTFE directly contribute to a more efficient, reliable, and cost-effective research operation.
Enabling Smooth Flow of Diverse Materials
Pharmaceutical research involves a wide array of materials, from fine powders that can clog a system to viscous liquids that can coagulate.
Low adhesion, combined with PTFE's low friction properties, allows these diverse materials to flow smoothly without interruption. This prevents blockages and ensures consistent process conditions.
Reducing Maintenance and Cleaning Cycles
Cleaning validation is a time-consuming and expensive part of pharmaceutical work. The more difficult a component is to clean, the higher the operational cost.
Because residues do not readily stick to PTFE, Clean-in-Place (CIP) cycles are faster and more effective. This reduces downtime, conserves cleaning agents, and lowers overall maintenance burdens.
Understanding the Trade-offs and Material Properties
While highly beneficial, low surface adhesion is one part of a larger picture. Its value is maximized when understood in the context of PTFE's other characteristics.
The Interplay with Chemical Inertness
Low adhesion alone would be insufficient if the valve material reacted with the process fluid. The true power of PTFE comes from the combination of its non-stick nature and its exceptional chemical inertness.
This dual benefit ensures that the sample not only avoids sticking to the valve but also remains chemically unchanged by it. It doesn't adhere, and it doesn't react.
Durability and High Cycle Life
A valve component must be mechanically robust. PTFE's durability allows it to withstand the repeated mechanical stress of valve actuation over many cycles.
This resilience means the valve maintains its integrity and sealing performance over time, preventing leaks and equipment malfunctions that could otherwise halt research.
Limitations to Consider
No material is a universal solution. While PTFE offers a superb balance of properties, it has thermal and mechanical limits. Its performance under extreme temperatures or high pressures must be considered during system design to avoid issues like material creep or deformation.
Making the Right Choice for Your Goal
Selecting the right valve material is a critical decision that should align with your primary objective.
- If your primary focus is high-purity API handling: PTFE's low adhesion and chemical inertness are essential to prevent sample loss and degradation, preserving the integrity of expensive compounds.
- If your primary focus is process automation and repeatability: The non-stick surface allows for consistent flow and complete cleaning, which is fundamental for validated, automated systems that require minimal human intervention.
- If your primary focus is cell culture or biologics: The smooth, sterile-friendly surface of PTFE minimizes cell shear and prevents biofilm formation, protecting sensitive biological materials.
Ultimately, choosing a PTFE diaphragm valve is an investment in the accuracy and reliability of your research outcomes.
Summary Table:
| Benefit of Low Adhesion | Impact on Pharmaceutical Research |
|---|---|
| Prevents Material Loss | Ensures accurate dosing of expensive APIs and compounds. |
| Reduces Cross-Contamination | Guarantees batch purity and valid experimental results. |
| Maintains Sterility | Inhibits biofilm formation and supports effective SIP cycles. |
| Improves Process Efficiency | Enables smooth flow, faster cleaning, and less downtime. |
Protect your most critical research with valves engineered for purity.
The right component is an investment in the accuracy and reliability of your outcomes. At KINTEK, we specialize in the precision manufacturing of high-performance PTFE components, including seals, liners, and custom labware.
We understand the stringent demands of the semiconductor, medical, and laboratory industries. Whether you need a standard solution or a custom-fabricated prototype for a specialized application, our focus is on delivering the chemical inertness, durability, and non-stick properties your processes require.
Ready to enhance your process integrity? Contact our experts today to discuss how our PTFE solutions can be tailored to your specific needs, from initial prototypes to high-volume production.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE and Nitrile Diaphragm Pump Components for Demanding Applications
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
People Also Ask
- What are the key advantages of using PTFE valves in the chemical industry? Enhance Safety and Purity
- What performance advantages do PTFE rotary shaft seals provide for machinery? Achieve Peak Efficiency & Reliability
- What are the key dielectric properties of PTFE that make it suitable for wires and cables? Ensuring Signal Integrity in Extreme Conditions
- What industries can benefit from PTFE-free bushings? Automotive, Aerospace, and Heavy Machinery Solutions
- What are the common physical properties of Expanded PTFE? Unlock Superior Sealing Performance
- What material is used to make PTFE bushings? The Ultimate Guide to PTFE's Performance
- How do PTFE seals help in reducing maintenance costs for ball valves? Slash Downtime & Boost Reliability
- What happens when clearance develops in a PTFE-lined bearing? A Guide to Catastrophic Failure



















