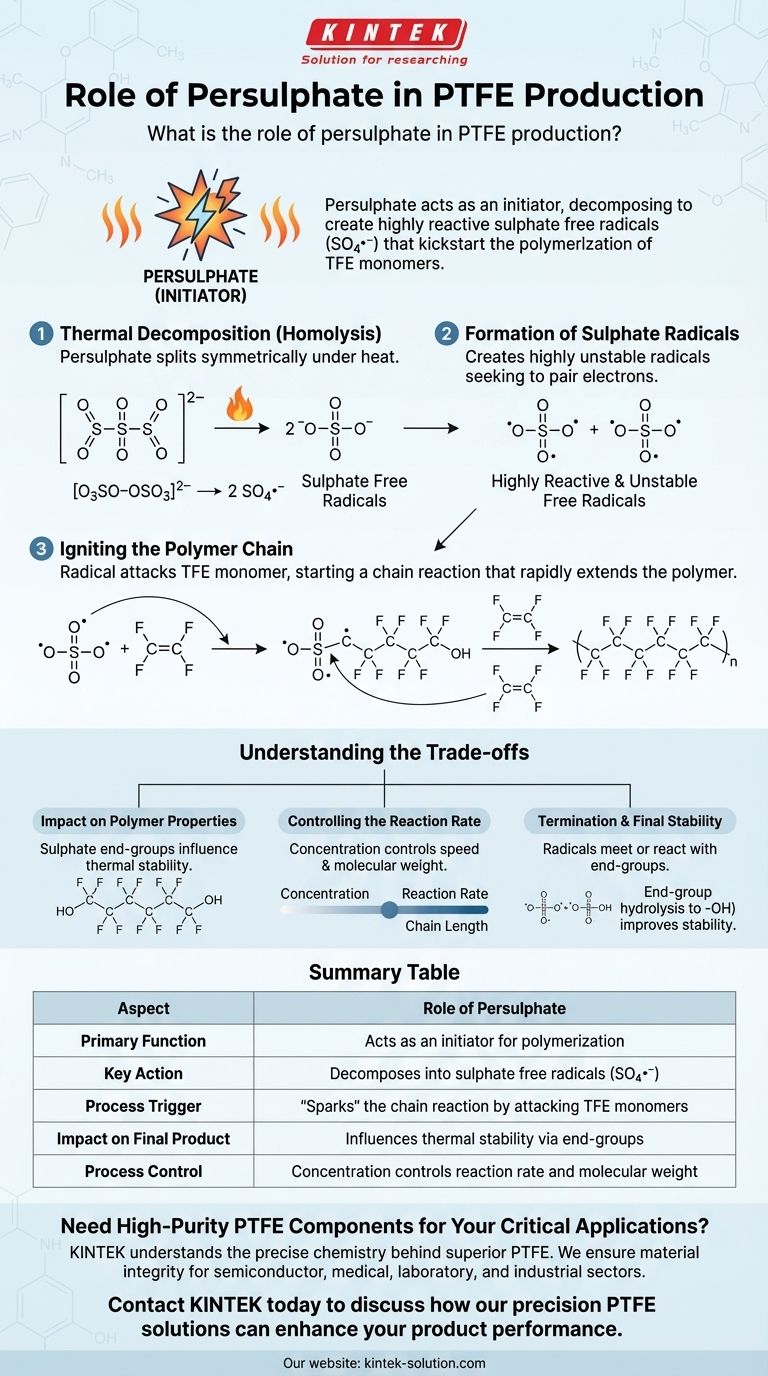
In the production of PTFE, the primary role of persulphate is to act as an initiator. It decomposes to create highly reactive sulphate free radicals, which are the essential catalysts that kickstart the chain-reaction polymerization of tetrafluoroethylene (TFE) monomers into the final polymer.
Persulphate doesn't become part of the main PTFE backbone; instead, it serves as the "spark" that ignites the chemical reaction. Its decomposition into free radicals is the critical first step that enables individual TFE molecules to link together into long, stable PTFE chains.

The Chemistry of Initiation
To understand persulphate's role, we must first understand the fundamental challenge of polymerization. The process involves converting many small, individual molecules (monomers) into a single, massive chain-like molecule (a polymer). This reaction does not happen spontaneously.
Step 1: Thermal Decomposition (Homolysis)
The process begins by applying energy, typically heat, to a persulphate salt (like ammonium or potassium persulphate) in water. This energy causes the persulphate molecule to split apart symmetrically in a process called homolysis.
[O3SO−OSO3]2− → 2 SO4•−
This is the most critical step. The original persulphate molecule is stable, but the resulting products are not.
Step 2: Formation of Sulphate Radicals
The result of this decomposition is the formation of two sulphate free radicals (SO4•−). A free radical is a molecule with an unpaired electron, which makes it extremely unstable and highly reactive.
This instability is the entire reason persulphate is used. The radical will aggressively seek to pair its lone electron by reacting with other nearby molecules.
Step 3: Igniting the Polymer Chain
The sulphate radical attacks the first available tetrafluoroethylene (TFE) monomer. It breaks the TFE's strong carbon-carbon double bond and attaches itself to one side, transferring the radical (the unpaired electron) to the other side of the monomer.
This creates a new, larger free radical. This new radical then attacks another TFE monomer, adding it to the chain and moving the radical to the new end. This process repeats thousands of times, rapidly extending the polymer chain.
Understanding the Trade-offs
While essential, the choice and concentration of an initiator like persulphate have direct consequences on the final product and process control.
Impact on Polymer Properties
The initiator fragments, in this case sulphate groups, remain at the ends of the finished PTFE polymer chains. These end-groups can influence the polymer's thermal stability and other properties.
Controlling the Reaction Rate
The concentration of persulphate is a key control lever. A higher concentration leads to more free radicals, which can increase the rate of polymerization. However, too many radicals can also lead to shorter polymer chains, affecting the material's final mechanical properties.
Termination and Final Stability
The reaction eventually terminates when two radicals meet or when the radicals react with the sulphate ester end-groups. As noted in the source material, these end-groups can later be hydrolyzed to form more stable hydroxyl (-OH) end-groups, which improves the final product's quality.
Key Takeaways for Process Understanding
- If your primary focus is process initiation: Persulphate is the indispensable starting agent. Without its ability to form free radicals under heat, the polymerization of TFE would not occur at a practical rate.
- If your primary focus is final polymer structure: Recognize that remnants of the persulphate initiator will be chemically bonded to the ends of the PTFE chains, influencing the material's bulk properties like thermal stability.
- If your primary focus is reaction control: The concentration and decomposition rate of persulphate are critical parameters used to manage the speed of polymerization and the molecular weight of the resulting PTFE.
Ultimately, persulphate functions as the precise chemical key needed to unlock the potential energy within TFE monomers, enabling their transformation into a stable and valuable polymer.
Summary Table:
| Aspect | Role of Persulphate |
|---|---|
| Primary Function | Acts as an initiator for polymerization |
| Key Action | Decomposes into sulphate free radicals (SO4•−) |
| Process Trigger | "Sparks" the chain reaction by attacking TFE monomers |
| Impact on Final Product | Influences thermal stability via end-groups |
| Process Control | Concentration controls reaction rate and molecular weight |
Need High-Purity PTFE Components for Your Critical Applications?
At KINTEK, we understand the precise chemistry behind superior PTFE. Our expertise in manufacturing high-performance PTFE seals, liners, and labware ensures the material integrity and reliability your projects demand.
We serve the semiconductor, medical, laboratory, and industrial sectors with custom fabrication from prototypes to high-volume orders.
Contact KINTEK today to discuss how our precision PTFE solutions can enhance your product performance.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- PTFE Chemical Solvent Sampling Spoon
- Customizable PTFE Crucibles for Laboratory and Industrial Applications
- Custom PTFE Bottles for Diverse Industrial Applications
People Also Ask
- What are the key properties that make Teflon widely applicable? Unlock Unmatched Chemical & Thermal Resistance
- What are the unique characteristics of porous ePTFE? Unlock Versatile Solutions for Complex Engineering Challenges
- What makes Teflon a versatile coating material? Unlock Superior Performance in Your Application
- How does PTFE benefit aerospace applications? Achieve Superior Performance in Extreme Environments
- What are the key properties of PTFE that make it ideal for industrial use? Unlock Unmatched Performance in Harsh Environments
- What is Polytetrafluoroethylene (PTFE) and what are its main types? Unlock High-Performance Solutions
- How does Nylon perform in chemical-heavy environments? A Guide to Its Strengths and Critical Weaknesses
- What is the chemical name for Teflon, and what is its abbreviation? Unveiling PTFE’s Unique Properties



















