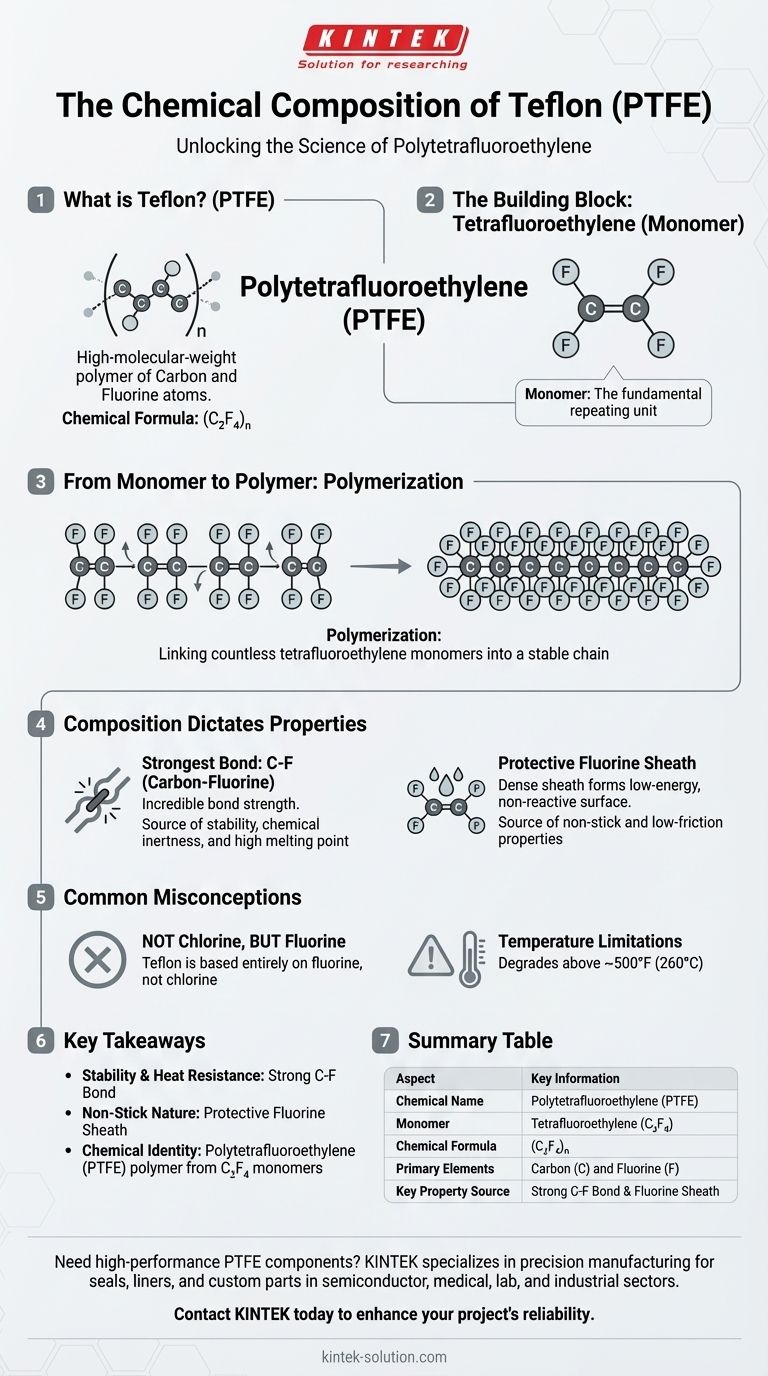
At its core, Teflon is the brand name for a synthetic polymer called Polytetrafluoroethylene (PTFE). This material is a high-molecular-weight compound composed exclusively of carbon and fluorine atoms. Its chemical formula is (C₂F₄)n, which signifies a long chain made of many repeating tetrafluoroethylene units.
The key to understanding Teflon is its structure: a long, stable backbone of carbon atoms completely shielded by a tightly packed sheath of fluorine atoms. This unique arrangement is the direct source of its famous non-stick, heat-resistant, and chemically inert properties.

The Fundamental Building Block: Tetrafluoroethylene
What is a Monomer?
In chemistry, a monomer is a single, small molecule that can chemically bond to other identical molecules to form a much larger molecule, known as a polymer. It is the fundamental repeating unit.
The C₂F₄ Molecule
The monomer for Teflon is tetrafluoroethylene (C₂F₄). This molecule consists of two carbon atoms joined by a double bond, with four fluorine atoms attached to the carbons.
From Monomer to Polymer: The Creation of PTFE
The Polymerization Process
Teflon is created through a process called polymerization. During this process, countless individual tetrafluoroethylene monomers are linked together end-to-end, breaking their double bonds to form long, stable single bonds with each other.
The Resulting Chemical Structure
The final result is Polytetrafluoroethylene (PTFE), a thermoplastic polymer. Its structure consists of a long, straight chain of carbon atoms, often referred to as a carbon "backbone."
Crucially, this carbon backbone is completely surrounded by fluorine atoms. This creates a dense, protective "sheath" around the carbon core.
The Chemical Formula: (C₂F₄)n
The chemical formula (C₂F₄)n elegantly represents this structure. The (C₂F₄) shows the repeating monomer unit, and the n signifies that this unit is repeated a very large number of times to form the polymer chain.
How Composition Dictates Teflon's Famous Properties
The Strength of the Carbon-Fluorine Bond
The chemical bond between carbon and fluorine is one of the strongest known in organic chemistry. This incredible bond strength makes the entire PTFE molecule highly stable and resistant to being broken down by heat or other chemicals.
This stability is the reason for Teflon's high melting point and its remarkable chemical inertness.
The Protective Fluorine Sheath
The fluorine atoms packed around the carbon backbone create a very low-energy, non-reactive surface. Because fluorine is the most electronegative element, this sheath effectively prevents other substances from forming bonds or sticking to the surface.
This molecular-level repulsion is the direct cause of Teflon's famous non-stick and low-friction properties.
Common Misconceptions to Avoid
Not Chlorine, but Fluorine
A common point of confusion is the specific halogen involved. Teflon's structure is based entirely on fluorine, not chlorine. Materials made with chlorine have vastly different properties.
Temperature Limitations
While highly heat-resistant, Teflon is not indestructible. At temperatures above approximately 500°F (260°C), PTFE can begin to degrade. Understanding this operational limit is critical for its safe and effective use.
Key Takeaways on Teflon's Composition
- If your primary focus is stability and heat resistance: The key is the exceptionally strong carbon-fluorine bond, which makes the molecule very difficult to break apart.
- If your primary focus is its non-stick nature: The reason is the protective sheath of fluorine atoms, which creates a low-energy surface that repels nearly everything.
- If your primary focus is its chemical identity: Remember its full name is Polytetrafluoroethylene (PTFE), a polymer built from repeating tetrafluoroethylene (C₂F₄) monomer units.
Ultimately, the extraordinary properties of Teflon arise directly from its simple yet robust chemical composition.
Summary Table:
| Aspect | Key Information |
|---|---|
| Chemical Name | Polytetrafluoroethylene (PTFE) |
| Monomer | Tetrafluoroethylene (C₂F₄) |
| Chemical Formula | (C₂F₄)n |
| Primary Elements | Carbon (C) and Fluorine (F) |
| Key Property Source | Strong Carbon-Fluorine Bond & Fluorine Atom Sheath |
Need high-performance PTFE components for your application?
At KINTEK, we specialize in the precision manufacturing of PTFE seals, liners, labware, and custom components. Our deep understanding of PTFE's chemical properties ensures we deliver parts that meet the highest standards for chemical resistance, thermal stability, and non-stick performance.
We serve the semiconductor, medical, laboratory, and industrial sectors with custom fabrication from prototypes to high-volume orders.
Contact KINTEK today to discuss how our PTFE solutions can enhance your project's reliability and efficiency.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Customizable PTFE Rods for Advanced Industrial Applications
People Also Ask
- Why is PTFE's chemical inertness important for aerospace applications? Ensuring Safety & Reliability in Demanding Environments
- How does PTFE benefit the food processing industry? Enhance Safety, Efficiency, and Purity
- What are the key properties of Polytetrafluoroethylene (PTFE)? Unlock Extreme Performance
- What are the limitations of PTFE materials? Understand the Key Trade-Offs Before You Spec
- What are some common uses of PTFE? Unlock Extreme Performance for Your Industry
- Is PTFE considered a metal or plastic? Understanding Its True Classification
- What are the uses of Teflon in personal care products? Enhance Makeup Longevity & Heat Protection
- What is ePTFE and how is it produced? Unlock the Power of Microporous PTFE



















