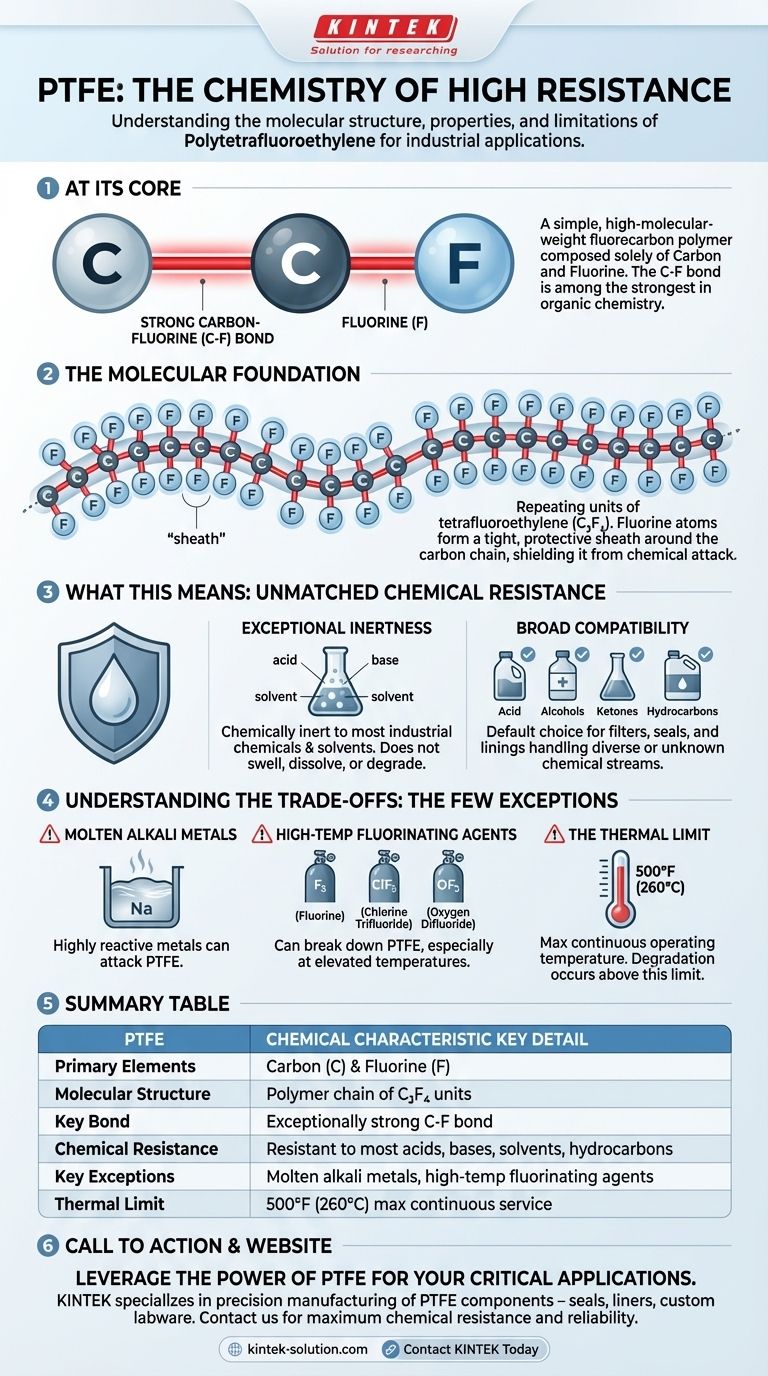
At its core, Polytetrafluoroethylene (PTFE) is a remarkably simple polymer. It is a fluorocarbon, a high-molecular-weight compound composed solely of two elements: carbon (C) and fluorine (F). This simple chemical makeup is the direct source of its extraordinary properties, including its famous chemical inertness and low-friction surface.
The immense strength of the bond between carbon and fluorine atoms is the single most important concept to understand. This bond makes PTFE one of the most chemically non-reactive and stable plastics known, defining its role in demanding industrial and chemical applications.

The Molecular Foundation of PTFE
The properties of PTFE are not magic; they are a direct result of its molecular structure. Understanding this structure reveals why it behaves the way it does.
A Polymer of Carbon and Fluorine
PTFE is a long chain, or polymer, built from repeating units of the tetrafluoroethylene monomer (C₂F₄). Imagine a long backbone made entirely of carbon atoms.
The Power of the Carbon-Fluorine Bond
Each carbon atom in the backbone is bonded to two fluorine atoms. The carbon-fluorine (C-F) bond is exceptionally strong and stable—one of the strongest single bonds in organic chemistry.
Because these bonds are so difficult to break, most chemicals simply lack the energy to react with the PTFE molecule.
A Protective Fluorine Sheath
The fluorine atoms are larger than the carbon atoms they are attached to. They effectively wrap around the carbon backbone, creating a tight, protective "sheath."
This sheath shields the carbon chain from potential chemical attack, further enhancing the material's inertness.
What This Means for Chemical Resistance
This unique molecular structure gives PTFE a chemical resistance profile that is nearly unmatched among polymers.
Exceptional Chemical Inertness
PTFE is essentially chemically inert and non-reactive to the vast majority of industrial chemicals and solvents. It does not swell, dissolve, or degrade when exposed to them.
Broad Chemical Compatibility
This inertness makes it highly compatible with a wide range of aggressive substances. This includes strong acids, bases, alcohols, ketones, hydrocarbons, and halogenated compounds.
This versatility makes it a default choice for components like filters, seals, and linings that must handle diverse or unknown chemical streams.
Understanding the Trade-offs: The Few Exceptions
While extraordinarily resistant, PTFE is not invincible. Its chemical stability has specific, well-defined limits that are critical to understand for safe and effective use.
Molten Alkali Metals
Highly reactive metals like molten sodium are one of the few substances that can attack PTFE.
High-Temperature Fluorinating Agents
Aggressive fluorinating chemicals can break down PTFE, especially at elevated temperatures. Key examples include turbulent liquid or gaseous fluorine, chlorine trifluoride (ClF₃), and oxygen difluoride (OF₂).
These substances are powerful enough to disrupt the strong carbon-fluorine bond that gives PTFE its stability.
The Thermal Limit
The chemical resistance of PTFE is generally rated up to its maximum continuous operating temperature of 500°F (260°C). Above this temperature, the material will begin to degrade, potentially releasing harmful fumes.
How to Apply This to Your Project
Understanding the fundamental chemistry of PTFE allows you to specify it with confidence.
- If your primary focus is broad chemical handling: PTFE is an excellent first choice for components like gaskets, seals, and pump parts due to its near-universal compatibility with common acids, bases, and solvents.
- If your primary focus is an extreme chemical environment: You must confirm that your application does not involve the specific exceptions, namely molten alkali metals or aggressive high-temperature fluorinating agents.
- If your primary focus is temperature resistance: PTFE performs reliably up to 500°F (260°C), but it should not be considered for applications that exceed this thermal limit.
By recognizing that PTFE's power comes directly from its simple carbon-fluorine structure, you can confidently leverage its unique strengths for your most demanding applications.
Summary Table:
| PTFE Chemical Characteristic | Key Detail |
|---|---|
| Primary Elements | Carbon (C) & Fluorine (F) |
| Molecular Structure | Polymer chain of tetrafluoroethylene (C₂F₄) units |
| Key Bond | Exceptionally strong Carbon-Fluorine (C-F) bond |
| Chemical Resistance | Resistant to most acids, bases, solvents, and hydrocarbons |
| Key Exceptions | Molten alkali metals and high-temperature fluorinating agents |
| Thermal Limit | 500°F (260°C) maximum continuous service temperature |
Leverage the Power of PTFE for Your Critical Applications
Understanding the chemistry of PTFE is the first step. Applying it effectively is the next. KINTEK specializes in the precision manufacturing of PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors. Whether you need a prototype or a high-volume production run, our expertise ensures your components deliver maximum chemical resistance and reliability.
Ready to specify the right material for your project? Contact KINTEK today to discuss your specific requirements and get a quote.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- What modifications can be made to PTFE for enhanced performance? Boost Wear Resistance & Strength with Fillers
- How did Teflon transition from industrial to consumer use? From Aerospace to Your Kitchen
- What are the additional beneficial properties of PTFE besides corrosion resistance? Leverage Its Full Potential for High-Performance Applications
- How does PTFE perform against various chemicals? Unmatched Chemical Resistance for Demanding Applications
- Why is PTFE considered safe for food and pharmaceutical applications? Ensuring Product Purity and Compliance
- What are the limitations of PTFE in its applications? Understanding Its Mechanical Weaknesses
- How does PTFE react to ammonia? Discover Its Superior Chemical Resistance
- What are some notable properties of PTFE? Discover the Extreme Performance of Teflon



















