At its core, Polytetrafluoroethylene's (PTFE) remarkable heat stability stems from its unique molecular structure. The incredibly strong carbon-fluorine bonds and the compact, interlocking sheath of fluorine atoms that protect the carbon backbone require significant thermal energy to disrupt, giving the material its high-performance characteristics.
PTFE's stability is not just about a high melting point. The true advantage lies in its wide and reliable continuous service temperature range, where it maintains its structural integrity and chemical inertness under conditions that cause most other plastics to fail.

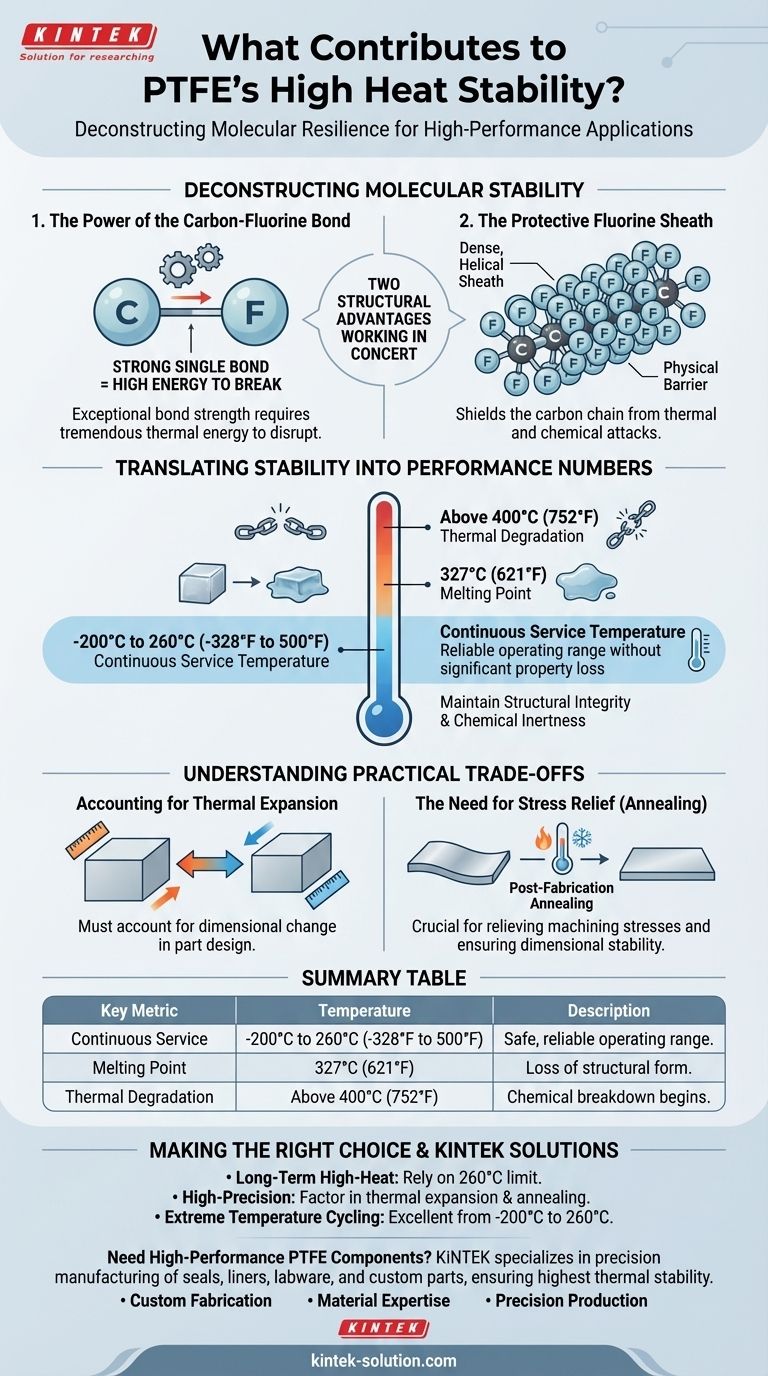
Deconstructing PTFE's Molecular Stability
To understand why PTFE performs so well under heat, we need to look at its chemical composition at the atomic level. Its resilience is not a single feature but the result of two key structural advantages working in concert.
The Power of the Carbon-Fluorine Bond
The bond between carbon and fluorine (C-F) is one of the strongest single bonds known in organic chemistry. This exceptional bond strength means it takes a tremendous amount of energy—in this case, thermal energy—to break the molecule apart.
This inherent strength is the primary reason PTFE does not easily degrade when exposed to heat.
The Protective Fluorine Sheath
Fluorine atoms are relatively large compared to the carbon atoms they are bonded to. In a PTFE polymer chain, these fluorine atoms form a dense, helical sheath around the carbon backbone.
This "interlocking" structure acts as a physical barrier, shielding the more vulnerable carbon chain from both thermal and chemical attacks.
Translating Stability into Performance Numbers
This molecular stability translates directly into measurable performance metrics that define PTFE's operational limits. It's crucial to distinguish between its melting point, service temperature, and degradation point.
Melting Point: 327°C (621°F)
This is the temperature at which PTFE transitions from a solid to a gel-like, viscous liquid. While it does not become a free-flowing liquid, it loses its structural form. This temperature represents a firm upper limit for any application.
Continuous Service Temperature: -200°C to 260°C (-328°F to 500°F)
This is the most critical number for real-world engineering. PTFE can operate continuously up to 260°C (500°F) without significant loss of its physical properties or structural integrity. Its ability to perform reliably across this vast range makes it exceptionally versatile.
Thermal Degradation: Above 400°C (752°F)
Thermal degradation, where the polymer chain itself begins to break down chemically, does not start until temperatures reach approximately 400°C. This demonstrates the material's immense inherent stability, well beyond its practical service limit.
Understanding the Practical Trade-offs
While chemically stable, using PTFE in high-temperature applications requires an understanding of its physical behaviors to ensure reliability and precision.
Accounting for Thermal Expansion
Like all materials, PTFE expands when heated and contracts when cooled. While it has a relatively low coefficient of thermal expansion for a polymer, this change in dimension must be accounted for in part design, especially for components with tight tolerances.
The Need for Stress Relief (Annealing)
Manufacturing processes like machining can introduce internal stresses into a PTFE part. When this part is later exposed to high temperatures, these stresses can release, causing warping or dimensional changes.
Post-fabrication annealing, a controlled heating and cooling process, is a critical step for relieving these stresses and ensuring the finished component remains dimensionally stable throughout its service life.
Making the Right Choice for Your Application
Selecting the right material requires matching its properties to your primary goal.
- If your primary focus is long-term high-heat operation: Rely on the continuous service temperature of 260°C (500°F) as your safe, reliable upper limit.
- If your primary focus is high-precision components: You must factor in thermal expansion and specify post-machining annealing to guarantee dimensional stability.
- If your primary focus is extreme temperature cycling: PTFE is an exceptional choice due to its ability to maintain its properties from cryogenic lows (-200°C) to high-heat (-260°C).
Ultimately, PTFE is the definitive choice when your application demands unwavering chemical and structural integrity across an exceptionally wide temperature range.
Summary Table:
| Key Metric | Temperature | Description |
|---|---|---|
| Continuous Service | -200°C to 260°C (-328°F to 500°F) | Safe, reliable operating range without significant property loss. |
| Melting Point | 327°C (621°F) | Temperature at which PTFE loses its structural form. |
| Thermal Degradation | Above 400°C (752°F) | Point where chemical breakdown of the polymer begins. |
Need High-Performance PTFE Components for Demanding Applications?
KINTEK specializes in the precision manufacturing of PTFE seals, liners, labware, and custom components. We understand the critical nature of thermal stability and dimensional precision. Our expertise ensures your parts are fabricated to the highest standards, including necessary post-machining annealing for stress relief, guaranteeing they perform reliably from cryogenic temperatures up to 260°C.
We serve the semiconductor, medical, laboratory, and industrial sectors with:
- Custom Fabrication: From prototypes to high-volume production runs.
- Material Expertise: Optimal selection and processing of PTFE for your specific thermal and chemical environment.
- Precision Production: Tight tolerances and dimensional stability are our priority.
Let us provide the reliable PTFE solution your application requires. Contact our engineering team today for a consultation.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Teflon Balls for Advanced Industrial Applications
People Also Ask
- What factors influence the coefficient of friction in PTFE materials? Optimize Performance for Your Application
- What are some lesser-known facts about Teflon? Uncover Its Hidden Role in Tech and Industry
- What are the mechanical properties of filled PTFE? Enhanced Wear Resistance and Strength for Demanding Applications
- How does PTFE perform against common acids and bases? Discover Unmatched Chemical Resistance
- How can PTFE adhere to another surface? Unlock Permanent Bonding with Chemical Etching
- What are common uses of PTFE? Unlock Versatility for Your Industry
- What makes PTFE unique compared to other engineering plastics? Unmatched Chemical & Thermal Resistance
- What is the role of valves in industrial piping systems? Ensure Safety, Control, and Efficiency



















