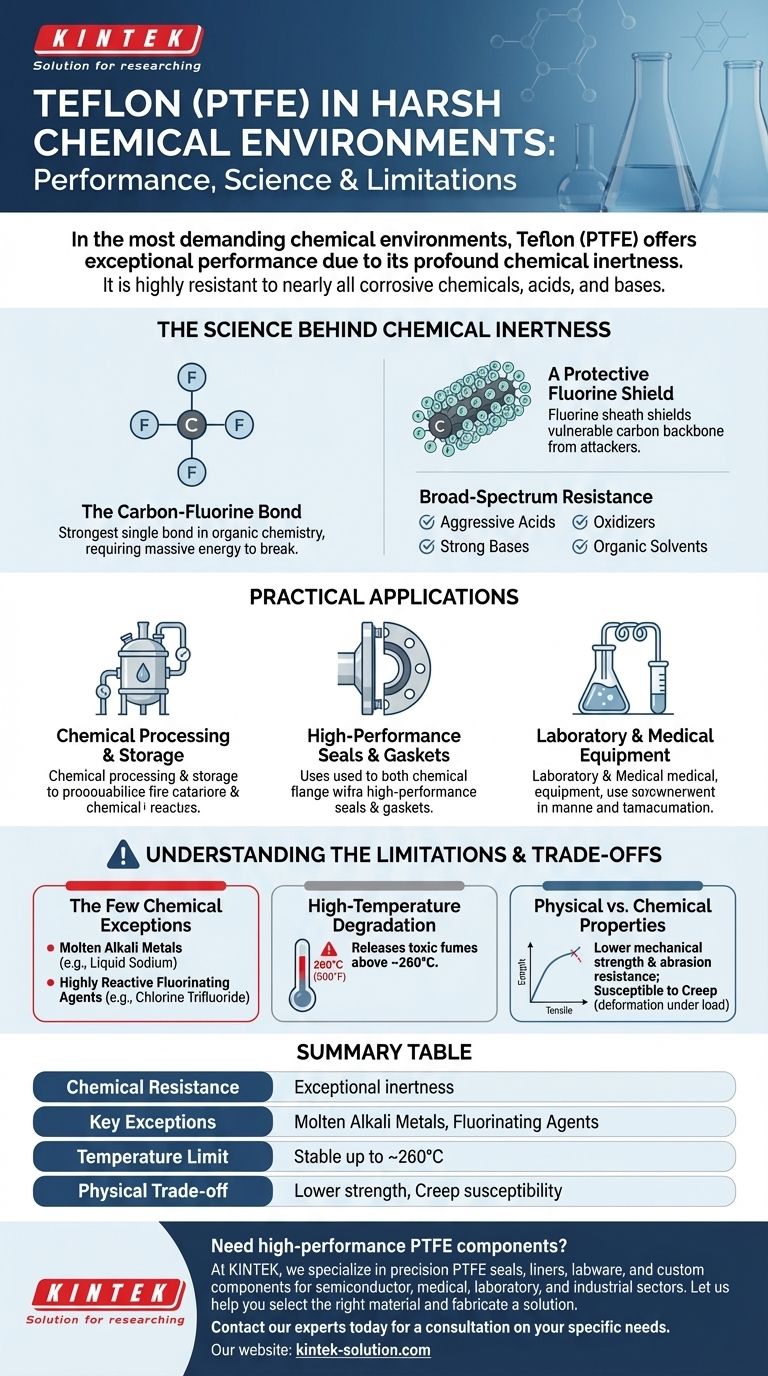
In the most demanding chemical environments, Teflon (PTFE) offers exceptional performance due to its profound chemical inertness. It is highly resistant to nearly all corrosive chemicals, acids, and bases, making it a default material choice for applications where chemical attack is a primary concern. Its reliability stems from a unique molecular structure that does not react with the vast majority of substances.
The core reason for Teflon's elite chemical resistance is the strength and stability of its carbon-fluorine bonds. This molecular structure creates a non-reactive surface, but it's critical to understand the few specific exceptions and physical trade-offs that define its operational limits.

The Science Behind Teflon's Chemical Inertness
To properly leverage Teflon, it's essential to understand why it is so non-reactive. Its performance is not a surface-level feature but a fundamental property of its molecular makeup.
The Carbon-Fluorine Bond
The foundation of Teflon's stability is the carbon-fluorine (C-F) bond, which is one of the strongest single bonds in organic chemistry. This bond is extremely difficult to break, meaning it requires a massive amount of energy to initiate a chemical reaction.
A Protective Fluorine Shield
The Teflon polymer chain consists of a carbon backbone completely surrounded by a dense, helical sheath of fluorine atoms. This fluorine sheath effectively shields the more vulnerable carbon backbone from potential chemical attackers, preventing them from getting close enough to react.
Broad-Spectrum Resistance
This combination of strong bonds and physical shielding makes Teflon inert to a vast range of substances. It remains unaffected by aggressive acids, strong bases, oxidizers, and nearly all organic solvents, ensuring material integrity and preventing contamination.
Practical Applications in Demanding Industries
Teflon's unique properties make it indispensable in sectors where material failure is not an option.
Chemical Processing and Storage
From vessel linings to pipes and fittings, Teflon is used to safely contain and transport highly corrosive materials. Its non-stick surface also helps prevent buildup and facilitates cleaning.
High-Performance Seals and Gaskets
As noted in industrial applications, Teflon seals provide durable, reliable leak protection in harsh chemical environments. It maintains its sealing capability and structural integrity even under prolonged exposure to aggressive media.
Laboratory and Medical Equipment
The inert nature of Teflon is critical in scientific and medical settings. It ensures that labware, tubing, or implantable devices do not react with chemicals or biological fluids, guaranteeing the purity of the process.
Understanding the Limitations and Trade-offs
While its chemical resistance is superior, no material is without limitations. An objective assessment requires acknowledging Teflon's specific vulnerabilities.
The Few Chemical Exceptions
Teflon's near-universal inertness has a few well-documented exceptions. It can be attacked by molten alkali metals (like liquid sodium) and certain highly reactive fluorinating agents (such as chlorine trifluoride and elemental fluorine at high temperatures).
High-Temperature Degradation
While Teflon has a high service temperature, it will begin to degrade above approximately 500°F (260°C). At these extreme temperatures, it can release toxic fumes, a critical safety consideration in material and system design.
Physical vs. Chemical Properties
Teflon's primary strength is chemical, not mechanical. It is a relatively soft material with lower tensile strength and abrasion resistance compared to many engineering plastics. It can also be susceptible to creep—a slow deformation under sustained load.
Making the Right Choice for Your Application
Selecting the correct material requires balancing chemical needs with mechanical and thermal demands.
- If your primary focus is broad chemical inertness: Teflon is an unparalleled choice for applications involving aggressive acids, bases, and solvents at moderate temperatures.
- If your application involves high mechanical loads or abrasion: You must account for Teflon's softness and potential for creep, possibly considering filled grades or alternative materials.
- If your environment involves molten alkali metals or potent fluorinating agents: You must explicitly avoid Teflon and seek a specialized alternative material.
Ultimately, understanding both the profound chemical resilience and the specific physical limitations of Teflon empowers you to use it effectively and safely.
Summary Table:
| Property | Performance in Harsh Chemical Environments |
|---|---|
| Chemical Resistance | Exceptional inertness to nearly all acids, bases, solvents, and oxidizers. |
| Key Exceptions | Vulnerable to molten alkali metals and certain fluorinating agents. |
| Temperature Limit | Stable up to ~260°C (500°F); degrades and emits fumes above this limit. |
| Physical Trade-off | Lower mechanical strength and abrasion resistance; susceptible to creep. |
Need high-performance PTFE components that can withstand your harsh chemical processes?
At KINTEK, we specialize in manufacturing precision PTFE seals, liners, labware, and custom components for the semiconductor, medical, laboratory, and industrial sectors. We understand the critical balance between chemical inertness and mechanical performance.
Let us help you select the right material and fabricate a solution—from prototypes to high-volume orders—that ensures reliability and safety in your most demanding applications.
Contact our experts today for a consultation on your specific needs.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- PTFE Chemical Solvent Sampling Spoon
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- What industries use PTFE machined parts and for what applications? Critical Components for Demanding Environments
- What are the key advantages of PTFE? Unmatched Performance for Extreme Environments
- Why is CNC machining preferred for Teflon parts over other methods? Unlock Precision & Complex Designs
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications
- Why is PTFE rod suitable for automotive applications? Boost Vehicle Performance & Durability



















