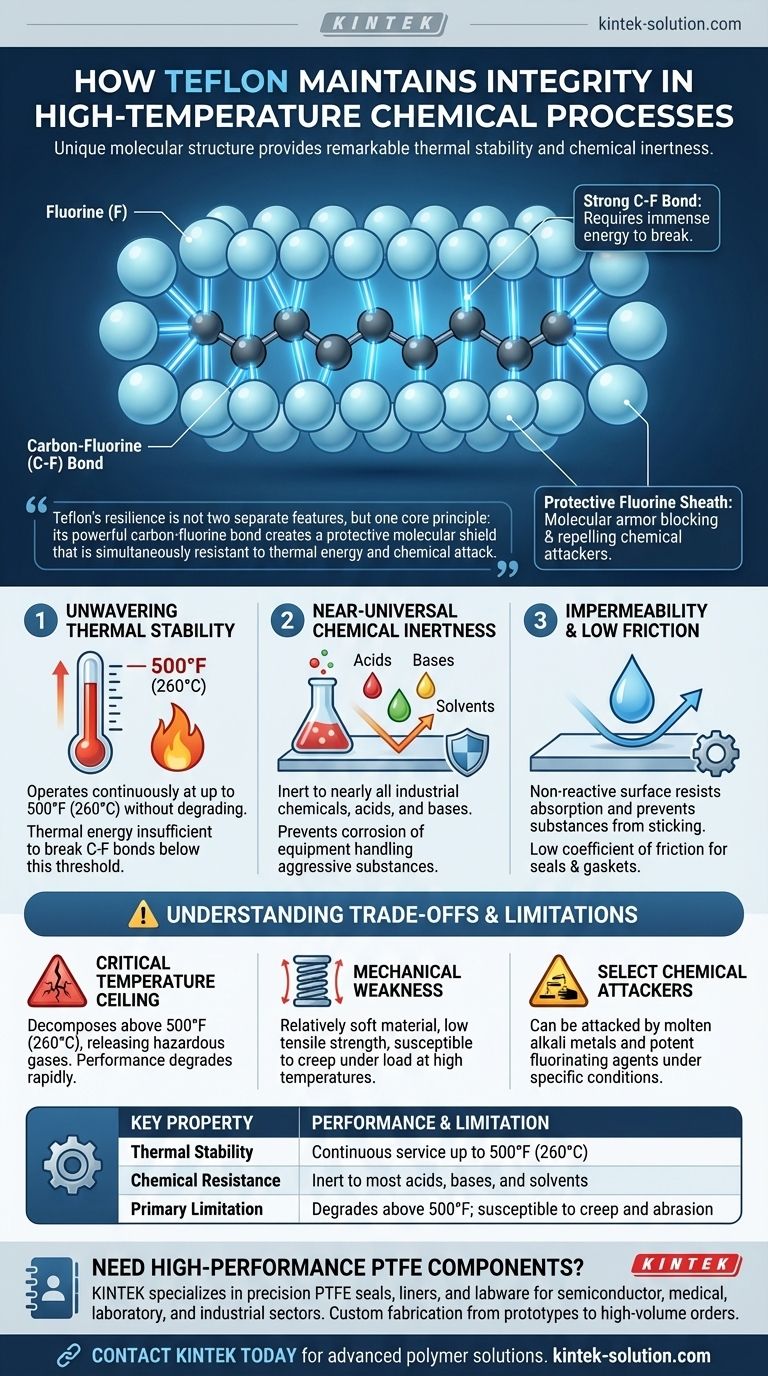
The integrity of Teflon in high-temperature chemical processes is a direct result of its unique molecular structure. The exceptionally strong bond between its carbon and fluorine atoms provides both remarkable thermal stability up to 500°F (260°C) and a non-reactive surface that is inert to nearly all industrial chemicals, acids, and bases.
Teflon's resilience is not two separate features, but one core principle: its powerful carbon-fluorine bond creates a protective molecular shield that is simultaneously resistant to thermal energy and chemical attack.

The Foundation of Resilience: The Carbon-Fluorine Bond
The performance of Polytetrafluoroethylene (PTFE), commercially known as Teflon, is not accidental. It stems from the fundamental nature of its chemical composition, which is one of the simplest and most robust in polymer science.
An Exceptionally Strong Molecular Link
The bond between a carbon atom and a fluorine atom is one of the strongest single bonds in organic chemistry. This immense strength means that a significant amount of energy is required to break the molecule apart.
A Protective Fluorine Sheath
In the Teflon polymer, the carbon chain backbone is completely encased by a tight sheath of fluorine atoms. This sheath effectively acts as molecular armor, physically blocking and electronically repelling potential chemical attackers from reaching the more vulnerable carbon backbone.
How This Structure Translates to Performance
This simple but powerful molecular design gives rise to the material properties that make Teflon invaluable in demanding industrial environments. It's how the microscopic structure creates macroscopic reliability.
Unwavering Thermal Stability
The strength of the C-F bond is the direct reason for Teflon's high heat tolerance. It can operate continuously at temperatures up to 500°F (260°C) without degrading or losing its essential properties. Below this threshold, thermal energy is simply insufficient to break the polymer chain.
Near-Universal Chemical Inertness
Because the carbon backbone is so well-protected by the fluorine sheath, Teflon is inert to the vast majority of chemicals. It can reliably seal or line equipment handling aggressive acids, bases, and solvents that would corrode metals and destroy other polymers.
Impermeability and Low Friction
The same non-reactive surface that resists chemical attack also prevents substances from sticking to or being absorbed by the material. This makes Teflon highly impermeable and gives it an extremely low coefficient of friction, a critical feature for components like seals and gaskets.
Understanding the Trade-offs and Limitations
While highly capable, Teflon is not an infallible solution. Understanding its operational boundaries is critical for safe and effective implementation in any process.
The Critical Temperature Ceiling
The 500°F (260°C) service temperature is a firm limit. Exceeding this temperature will cause the polymer to decompose, which can release hazardous gases. Its performance degrades rapidly as it approaches this ceiling.
Mechanical Weakness
Teflon is a relatively soft material. While its chemical resistance is elite, it does not possess high tensile strength or resistance to abrasion and can be susceptible to creep under sustained load, especially at higher temperatures.
A Select Few Chemical Attackers
Although its chemical resistance is exceptionally broad, it is not absolute. Teflon can be attacked by highly reactive substances like molten alkali metals and some potent fluorinating agents under specific conditions.
Making the Right Choice for Your Application
To leverage Teflon's strengths, you must align its properties with your specific operational goals.
- If your primary focus is sealing against corrosive media below 500°F (260°C): Teflon is an industry-standard choice for gaskets, valve seats, and linings due to its chemical inertness.
- If your application involves high-temperature steam or refined hydrocarbons: Teflon's thermal stability makes it a reliable material for gland fillers and seals in these demanding processes.
- If your process involves significant abrasive wear or temperatures well above 500°F (260°C): You must consider alternative materials like specific metals, ceramics, or other high-performance polymers.
Understanding the fundamental chemistry of Teflon empowers you to use it effectively where it excels and avoid it where its limitations would pose a risk.
Summary Table:
| Key Property | Performance & Limitation |
|---|---|
| Thermal Stability | Continuous service up to 500°F (260°C) |
| Chemical Resistance | Inert to most acids, bases, and solvents |
| Primary Limitation | Degrades above 500°F; susceptible to creep and abrasion |
Need High-Performance PTFE Components for Demanding Applications?
KINTEK specializes in manufacturing precision PTFE components—including seals, liners, and labware—for the semiconductor, medical, laboratory, and industrial sectors. Our custom fabrication services, from prototypes to high-volume orders, ensure you get parts that deliver unwavering integrity in high-temperature, corrosive environments.
Contact KINTEK today to discuss your specific requirements and leverage our expertise in advanced polymer solutions.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Bottles for Diverse Industrial Applications
- Customizable PTFE Seals Filter Holders for Versatile Applications
People Also Ask
- What are the primary applications of Teflon? Leverage Its Unique Properties for Your Industry
- What are the primary applications of PTFE? Unlocking High-Performance Solutions
- What industrial applications does PTFE have? Unlock Performance in Extreme Environments
- Why is chemical compatibility important when choosing a PTFE-coated septum? Avoid Sample Contamination and Data Loss
- What are the unique properties of PTFE that make it commercially valuable? Unlock Unmatched Performance



















