Polytetrafluoroethylene (PTFE) demonstrates its strong chemical resistance due to the unique stability and structure of its core molecular bonds. It is one of the most chemically inert polymers known, remaining completely unaffected by nearly all corrosive liquids, acids, bases, and solvents. This exceptional resilience makes it a critical material in industries where exposure to aggressive chemicals is constant.
The source of PTFE's legendary chemical resistance is twofold: the immense strength of the carbon-fluorine (C-F) bond and the way the fluorine atoms form a tight, protective helical sheath around the polymer's carbon backbone, effectively shielding it from attack.

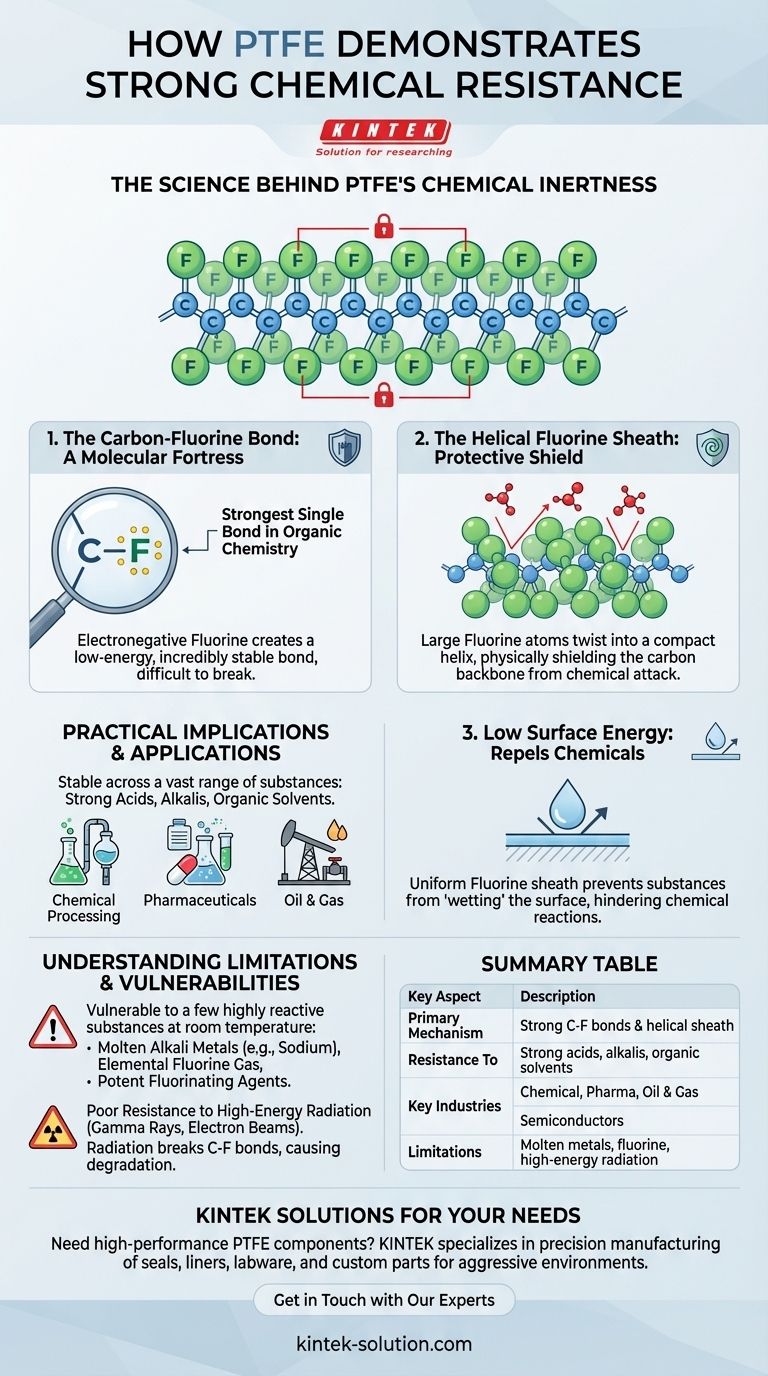
The Science Behind PTFE's Chemical Inertness
To understand why PTFE is so non-reactive, we must look at its structure at the molecular level. Its properties are not accidental; they are a direct result of its chemical composition and physical arrangement.
The Carbon-Fluorine Bond: A Fortress at the Molecular Level
The bond between carbon and fluorine atoms is one of the strongest single bonds in all of organic chemistry.
Fluorine is the most electronegative element, meaning it pulls bonding electrons very tightly towards itself. This creates an extremely stable, low-energy, and non-polar bond that is incredibly difficult for other chemicals to break apart.
The Helical Fluorine Sheath
The fluorine atoms are significantly larger than the carbon atoms they are bonded to. This forces the long chain of carbon atoms—the polymer's "backbone"—to twist into a compact helical shape.
This twist creates a perfect, seamless sheath of fluorine atoms covering the entire molecule. This sheath physically blocks chemicals from ever reaching the more vulnerable carbon backbone, preventing any potential reaction from initiating.
Low Surface Energy
This uniform, non-polar fluorine sheath gives PTFE an extremely low surface energy. This is why materials, including aggressive chemicals, have difficulty "wetting" its surface. For a chemical reaction to occur, intimate contact is required, and PTFE's structure inherently prevents this.
Practical Implications of Extreme Resistance
This molecular stability translates directly into reliable performance in the most demanding real-world applications.
Performance Across a Wide Spectrum
Because of its inert molecular structure, PTFE is stable when exposed to a vast range of substances. This includes highly corrosive agents like strong acids, alkalis (bases), and nearly all organic solvents. There are no known solvents that can dissolve PTFE at room temperature.
Essential for Demanding Industries
This unmatched resistance makes PTFE indispensable in sectors like chemical processing, pharmaceuticals, and oil and gas. It is used for lining vessels, seals, gaskets, and tubing where failure due to chemical attack would be catastrophic.
Understanding the Limitations and Weaknesses
While its resistance is remarkable, PTFE is not invincible. An objective assessment requires understanding its few specific vulnerabilities.
The Few Chemical Exceptions
At room temperature, PTFE is only affected by a very small number of highly reactive substances. These include molten alkali metals (like sodium), elemental fluorine gas, and extremely potent fluorinating agents such as chlorine trifluoride.
Vulnerability to High-Energy Radiation
PTFE has a relatively poor resistance to high-energy radiation, such as gamma rays or electron beams. This type of energy is powerful enough to physically break the strong carbon-fluorine bonds, causing the polymer chain to break down and lose its structural integrity and desirable properties.
Making the Right Choice for Your Application
Knowing the specific strengths and weaknesses of PTFE allows for precise material selection.
- If your primary focus is handling aggressive chemicals: PTFE is the industry benchmark and one of the most reliable options available, inert to virtually all common acids, bases, and solvents.
- If your application involves a high-radiation environment: You must avoid standard PTFE, as its molecular structure will degrade, leading to material failure.
- If you are working with molten alkali metals or niche fluorinating agents: Seek a specialized material, as these are the few known chemicals that can chemically attack and degrade PTFE.
Understanding the molecular basis of PTFE's resilience empowers you to deploy it with confidence in the world's harshest chemical environments.
Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Mechanism | Strong carbon-fluorine (C-F) bonds and a protective helical sheath of fluorine atoms. |
| Resistance To | Strong acids, alkalis, and nearly all organic solvents. |
| Key Industries | Chemical processing, pharmaceuticals, oil & gas, semiconductors, and laboratories. |
| Limitations | Vulnerable to molten alkali metals, elemental fluorine, and high-energy radiation. |
Need high-performance PTFE components that can withstand your most aggressive chemical processes?
At KINTEK, we specialize in the precision manufacturing of PTFE seals, liners, labware, and custom components. Whether you require standard parts or custom-fabricated solutions—from prototypes to high-volume production—our expertise ensures your equipment operates reliably in the harshest environments.
Contact us today to discuss your specific requirements and let our solutions enhance your operational safety and efficiency.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- What are some alternative materials to Teflon and their properties? Find the Right High-Performance Polymer for Your Application
- How is PTFE used in industrial applications? Solve Extreme Chemical, Thermal & Friction Challenges
- How was PTFE discovered and what were its initial findings? A Serendipitous Breakthrough in Material Science
- Why is PTFE chemically resistant and what applications benefit from this? Discover the Ultimate Material for Harsh Environments
- What is the coefficient of friction of PTFE? Unlocking Its Slippery Secrets for Your Designs
- How does the friction of Teflon compare to other materials? Discover the Benchmark for Low Friction
- What makes PTFE suitable for electrical applications? Superior Insulation for Demanding Environments
- What overall advantages does PTFE provide? Achieve Peak Performance in Harsh Environments



















