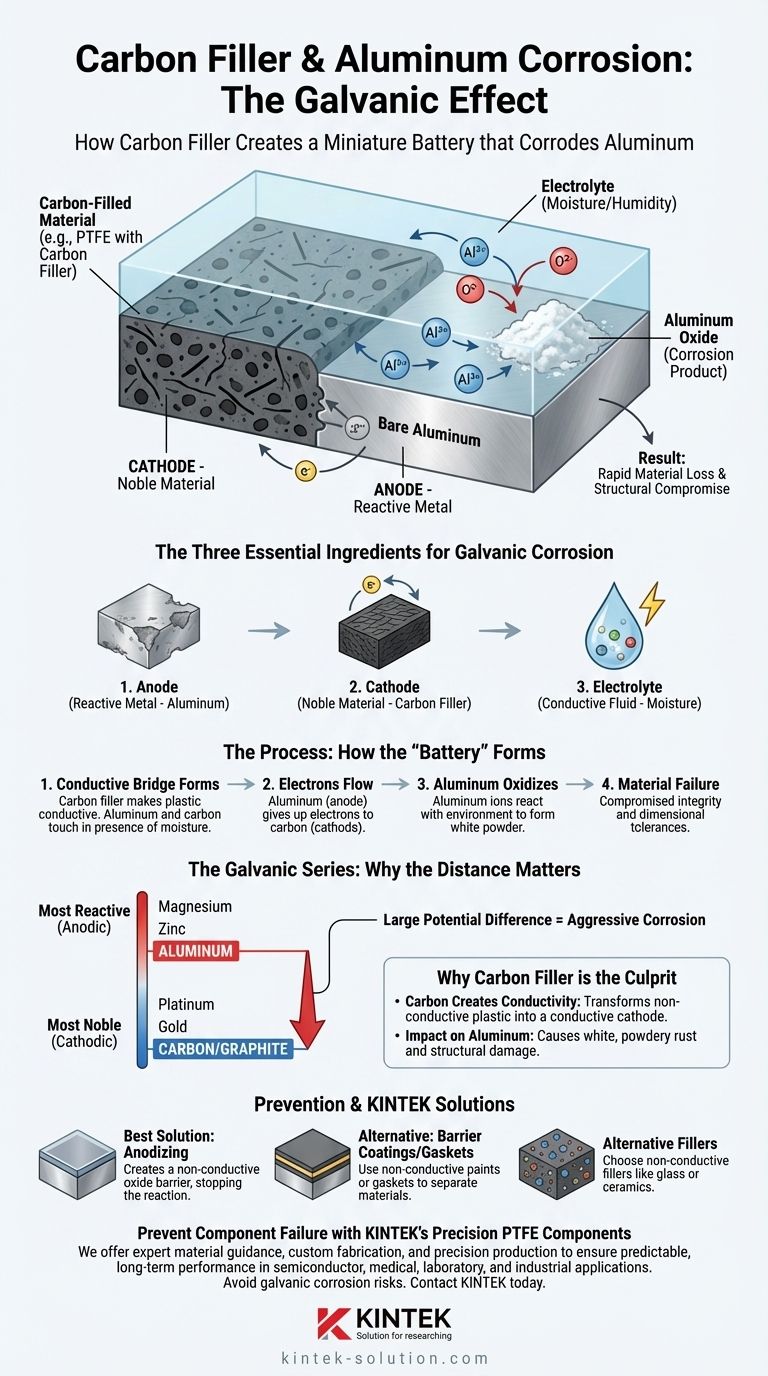
At its core, carbon filler causes corrosion with aluminum through an electrochemical process known as galvanic corrosion. Because carbon is electrically conductive and behaves like a noble metal, it creates a miniature battery when it touches the more reactive aluminum in the presence of even a small amount of moisture. This "battery" actively sacrifices the aluminum, converting it into aluminum oxide.
The fundamental issue is that combining a conductive, carbon-filled material with bare aluminum creates the perfect conditions for a corrosive reaction. The carbon acts as a cathode, the aluminum acts as an anode, and environmental moisture acts as the electrolyte, aggressively degrading the aluminum surface.

The Science of Galvanic Corrosion
To understand why this specific material combination is problematic, you must first understand the principles of galvanic corrosion. This type of corrosion occurs when three specific conditions are met.
The Three Essential Ingredients
Galvanic corrosion requires an anode (the more reactive metal that corrodes), a cathode (the less reactive, or noble, metal that is protected), and an electrolyte (a conductive fluid, like water). When these are connected, an electrical circuit forms, and the anode begins to degrade rapidly.
The Role of the Galvanic Series
Materials can be ranked by their electrochemical potential in a "galvanic series." Metals at the top, like aluminum and zinc, are highly reactive and willing to give up electrons (anodes). Materials at the bottom, like gold, platinum, and carbon/graphite, are very stable and noble (cathodes).
The farther apart two materials are on this series, the greater the electrical potential between them and the faster the anode will corrode. Carbon and aluminum are very far apart, creating a highly aggressive corrosion cell.
How the "Battery" Forms
When carbon-filled plastic touches aluminum, the aluminum becomes the anode and the carbon becomes the cathode. Any ambient moisture, condensation, or humidity can act as the electrolyte, completing the circuit.
Electrons flow from the aluminum to the carbon. This process dissolves the aluminum, which then reacts with the environment to form aluminum oxide—a distinctive white, powdery rust.
Why Carbon Filler is the Culprit
The polymer itself, such as PTFE, is typically an excellent electrical insulator and would not cause this issue. The problem is introduced entirely by the filler material chosen to enhance its properties.
Carbon Creates Conductivity
Fillers are added to polymers to improve properties like strength, thermal conductivity, or wear resistance. While effective, carbon filler transforms the non-conductive plastic into a conductive composite. This conductivity is precisely what enables it to act as a cathode against the aluminum.
The Impact on the Aluminum
The result of this galvanic reaction is the visible formation of a white aluminum oxide layer on the aluminum surface at the point of contact. This isn't just a cosmetic issue; it represents a loss of material that can compromise the structural integrity and dimensional tolerances of the aluminum component.
Common Pitfalls and Mitigation Strategies
Preventing this form of corrosion is critical for the long-term reliability of any assembly involving these materials. Simply hoping the environment stays dry is not a viable engineering solution.
Misunderstanding the Electrolyte
A common mistake is assuming an electrolyte must be a significant body of liquid like saltwater. In reality, normal atmospheric humidity is often sufficient to create a thin, conductive film of moisture that will activate the galvanic cell.
The Most Common Solution: Anodizing
The most effective way to prevent this corrosion is to anodize the aluminum component. Anodizing is an electrochemical process that grows a controlled layer of hard, non-conductive aluminum oxide on the surface.
This factory-grown layer is far more robust than the corrosion product and acts as a perfect electrical insulator. By breaking the electrical path between the carbon and the base aluminum, it completely stops the galvanic reaction from ever starting.
Alternative Mitigation Methods
Other options include applying a barrier coating or paint to the aluminum surface or using a non-conductive gasket to separate the two materials. In the design phase, one could also specify a polymer with a non-conductive filler (like glass or certain ceramics) if it meets the application's other requirements.
Making the Right Choice for Your Design
Understanding this interaction is key to preventing premature component failure. Your approach should be dictated by your specific design constraints and goals.
- If your primary focus is protecting an existing assembly: Anodizing the aluminum component is the most reliable and widely accepted solution to prevent galvanic corrosion from carbon fillers.
- If your primary focus is designing a new system: Evaluate whether a non-conductive filler, such as glass fiber, could meet your mechanical and thermal needs without introducing corrosion risk.
- If direct material changes are not possible: Ensure a durable, non-conductive barrier coating or sealant is applied and maintained at the interface between the carbon-filled material and the aluminum.
Ultimately, successful engineering relies on anticipating and managing material incompatibilities to ensure predictable, long-term performance.
Summary Table:
| Element | Role in Corrosion | Key Characteristic |
|---|---|---|
| Carbon Filler | Acts as the Cathode | Electrically conductive, noble material |
| Aluminum | Acts as the Anode | Reactive metal that corrodes (oxidizes) |
| Moisture | Acts as the Electrolyte | Enables the electrical circuit, even humidity |
| Result | Galvanic Corrosion | Formation of white, powdery aluminum oxide |
Prevent Component Failure with KINTEK's Precision PTFE Components
Understanding material incompatibility is crucial for the long-term reliability of your assemblies in the semiconductor, medical, laboratory, and industrial sectors. If your design involves aluminum and conductive materials, let KINTEK provide the solution.
We specialize in manufacturing high-performance PTFE components—including seals, liners, and custom labware. While carbon fillers can introduce corrosion risks, we can guide you on material selection or fabricate parts with alternative, non-conductive fillers (like glass) to meet your mechanical needs without compromising your aluminum components.
Our expertise ensures your designs perform predictably. We offer:
- Custom Fabrication: From prototypes to high-volume orders.
- Material Guidance: Helping you select the right polymer and filler for your specific application to avoid galvanic corrosion.
- Precision Production: Ensuring every component meets exact tolerances for reliable performance.
Don't let material incompatibility compromise your system. Contact KINTEK today to discuss your project requirements and explore corrosion-resistant solutions.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- PTFE Chemical Solvent Sampling Spoon
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- How is PTFE packing utilized in the food industry? Ensuring Food Safety and Efficiency
- What are the drawbacks of using PTFE for sealing needs? The Critical Trade-offs in Chemical vs. Mechanical Performance
- What are the two main manufacturing methods for Teflon washers? Choose the Right Process for Your Project
- What pressure ranges are common in oil and gas applications, and how do PTFE seals perform? Reliable Sealing from 1,500 to 25,000 PSI
- What are the chemical resistance properties of PTFE? Unmatched Inertness for Demanding Applications
- How is PTFE processed into useful shapes? Mastering the Unique Compression & Sintering Method
- What are some common defects of PTFE gaskets and how can they be managed? Mitigate Creep & Cold Flow for Reliable Seals
- Why should traditional lubricants not be used with PTFE-lined bearings? Avoid Premature Failure and High Friction


















