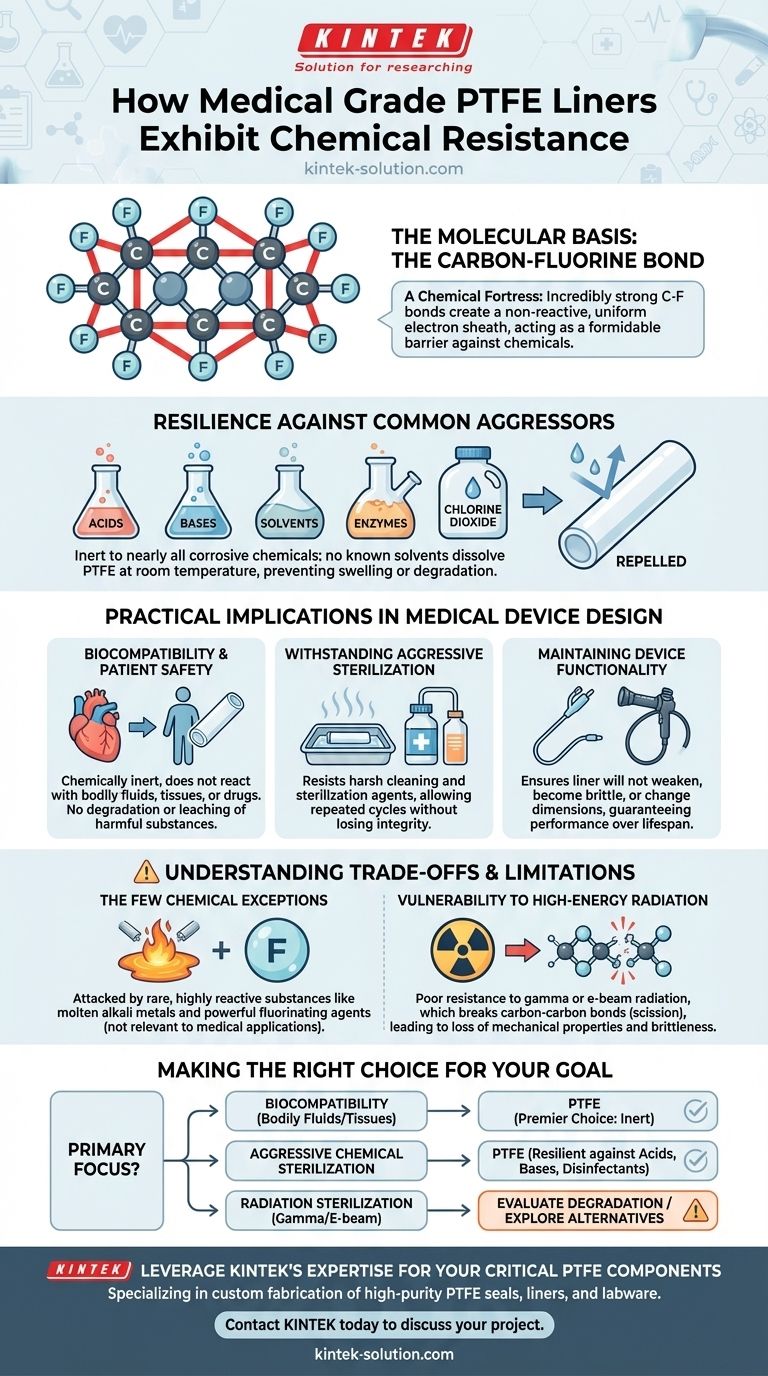
Medical Grade PTFE liners achieve their exceptional chemical resistance through their unique and stable molecular structure. The incredibly strong bonds between carbon and fluorine atoms create a non-reactive, protective sheath around the polymer's core. This makes the material functionally inert to nearly all acids, bases, solvents, and enzymes, ensuring the liner's structural integrity and functionality are maintained even after exposure to aggressive substances or frequent sterilization cycles.
The profound chemical inertness of PTFE isn't just a feature; it is the foundation of its reliability in critical medical devices. This stability stems from the powerful carbon-fluorine bond, which effectively shields the material from chemical attack, with only a few, highly specific exceptions.

The Molecular Basis of PTFE's Inertness
To understand why Medical Grade Polytetrafluoroethylene (PTFE) is so robust, we must look at its chemistry. The material's resilience is not an accident but a direct result of its atomic composition and structure.
The Carbon-Fluorine Bond: A Chemical Fortress
The strength of PTFE lies in the bond between its carbon and fluorine atoms. Fluorine is the most electronegative element, meaning it holds its electrons very tightly.
When bonded to carbon, it creates a uniform, dense sheath of electrons around the carbon backbone of the polymer. This sheath is exceptionally stable and non-polar, acting as a formidable barrier that prevents other chemicals from getting close enough to react.
Resilience Against Common Aggressors
This molecular structure makes PTFE unaffected by the vast majority of corrosive chemicals used in medical and industrial settings.
It is highly resistant to strong acids, alkalis, alcohols, and aggressive cleaning agents like chlorine dioxide. Furthermore, there are no known solvents that can dissolve PTFE at room temperature, which prevents chemical-induced swelling or degradation.
Practical Implications in Medical Device Design
This extreme chemical resistance is not just an academic property; it has direct and critical consequences for the performance and safety of medical devices.
Ensuring Biocompatibility and Patient Safety
Because PTFE is chemically inert, it does not react with bodily fluids, tissues, or administered drugs. This prevents the liner from degrading and leaching harmful substances into the patient's system, making it an outstandingly biocompatible material.
Withstanding Aggressive Sterilization Protocols
Medical devices must be sterilized to prevent infection, often using harsh chemicals. PTFE's ability to resist these agents means devices can undergo repeated, aggressive cleaning and sterilization cycles without losing their structural integrity or functional properties.
Maintaining Device Functionality
In devices like catheters or endoscopes, the liner's integrity is paramount. PTFE's chemical resistance ensures that the liner will not weaken, become brittle, or change its dimensions when exposed to various substances, guaranteeing the device performs as designed throughout its intended lifespan.
Understanding the Trade-offs and Limitations
While PTFE is the most chemically resistant plastic known, no material is without limitations. Objectivity requires acknowledging its specific vulnerabilities, even if they are rarely encountered in typical medical scenarios.
The Few Chemical Exceptions
PTFE can be attacked by a very small number of highly reactive substances. These include molten alkali metals (like sodium) and powerful fluorinating agents, such as elemental fluorine and chlorine trifluoride. These conditions are extreme and not relevant to medical applications.
Vulnerability to High-Energy Radiation
The most significant limitation in a medical context is PTFE's relatively poor resistance to high-energy radiation. Sterilization methods like gamma or e-beam radiation can break the carbon-carbon bonds in the polymer chain.
This process, known as scission, reduces the material's molecular weight, which can lead to a loss of mechanical properties like tensile strength and make the material brittle. This is a critical factor to consider during the device design and manufacturing process.
Making the Right Choice for Your Goal
Ultimately, material selection is about matching a material's properties to the specific demands of an application.
- If your primary focus is compatibility with bodily fluids and tissues: PTFE's inertness makes it a premier choice, as it will not react with or degrade within the biological environment.
- If your device requires frequent, aggressive chemical sterilization: PTFE's resilience against acids, bases, and common disinfectants ensures it will maintain its integrity through many cleaning cycles.
- If you plan to use radiation sterilization (gamma or e-beam): You must carefully evaluate the potential for PTFE degradation and consider the dose effects, or explore alternative materials or sterilization methods.
Understanding both the profound chemical strengths and the specific limitations of PTFE is the key to leveraging its power effectively in demanding medical applications.
Summary Table:
| Key Aspect | Explanation |
|---|---|
| Molecular Basis | Strong carbon-fluorine bonds create a protective electron sheath, making the material inert. |
| Resistance Profile | Highly resistant to virtually all acids, bases, solvents, and alcohols. |
| Medical Benefits | Ensures biocompatibility, withstands aggressive sterilization, and maintains device functionality. |
| Key Limitation | Vulnerable to degradation from high-energy radiation sterilization (e.g., gamma, e-beam). |
Leverage KINTEK's Expertise for Your Critical PTFE Components
For semiconductor, medical, laboratory, and industrial applications, the precise chemical resistance of your PTFE components is non-negotiable. KINTEK specializes in the custom fabrication of high-purity PTFE seals, liners, and labware, ensuring your devices meet the highest standards of performance and safety.
We partner with you from prototype to high-volume production, guaranteeing that your components deliver the reliable chemical inertness your application demands.
Contact KINTEK today to discuss your project requirements and discover how our precision manufacturing can enhance your product's reliability.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Customizable PTFE Seals Filter Holders for Versatile Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- What makes the PTFE bottle durable? Unmatched Chemical & Thermal Stability for Demanding Applications
- What material is the PTFE bottle made from? Discover the Benefits of 100% Virgin PTFE
- What are the unique properties of PTFE that make it commercially valuable? Unlock Unmatched Performance
- What are the primary applications of PTFE? Unlocking High-Performance Solutions
- Why are PTFE vials considered environmentally friendly? Reduce Lab Waste with Durable Reusables



















