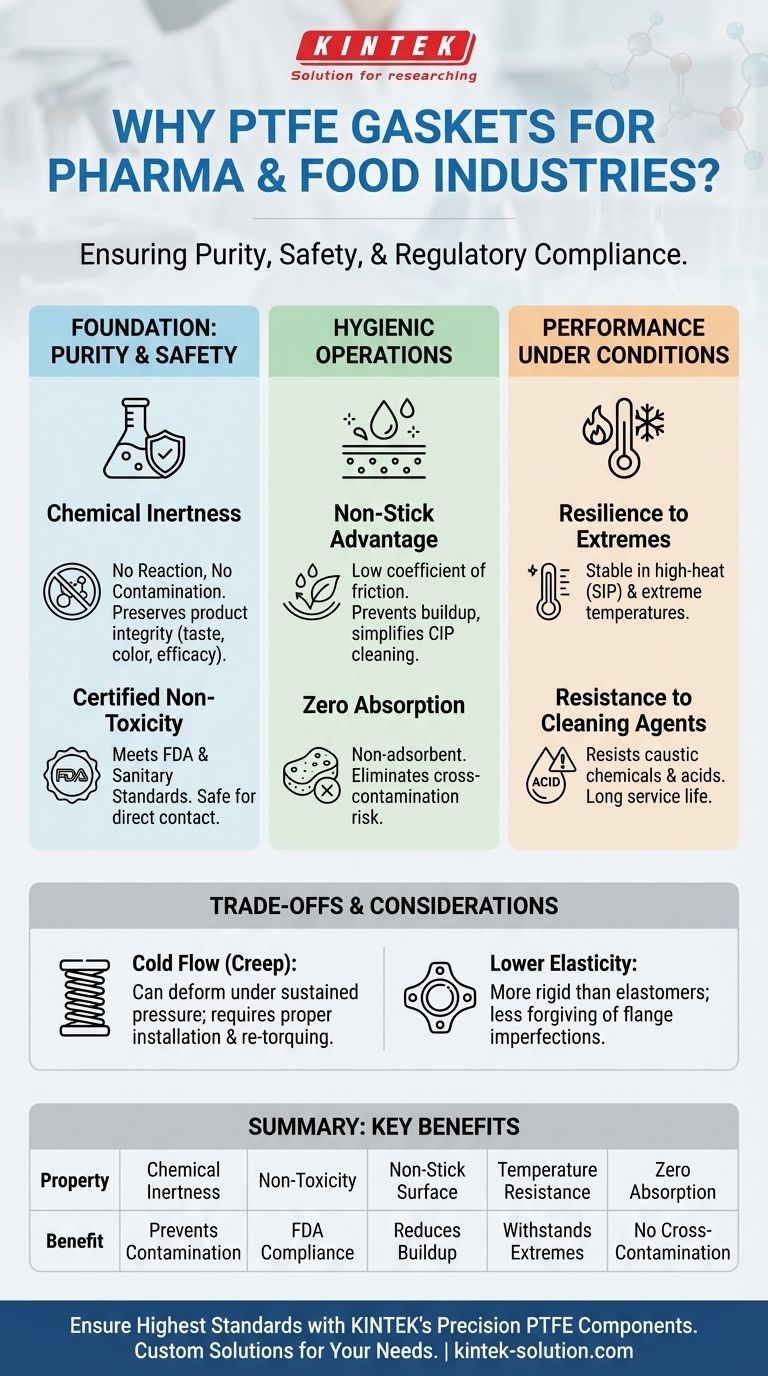
In short, PTFE gaskets are standard in pharmaceutical and food industries because their material properties directly address the highest priorities of these sectors: product purity, consumer safety, and regulatory compliance. Their unique combination of being chemically inert, non-toxic, and non-stick prevents contamination, ensures the product remains unaltered, and meets stringent FDA and other sanitary standards.
The core function of a gasket in a food or pharmaceutical line is not just to prevent leaks, but to act as a critical control point for product safety. PTFE is chosen because its inherent material properties—purity, non-reactivity, and resistance to buildup—make it one of the safest and most reliable materials for direct and indirect product contact.

The Foundation: Ensuring Product Purity and Safety
In regulated industries, any material that touches the product is considered a potential source of contamination. PTFE's fundamental chemistry provides a powerful safeguard against this risk.
Chemical Inertness: No Reaction, No Contamination
PTFE (polytetrafluoroethylene) is one of the most non-reactive substances known. It does not react with, leach into, or degrade when exposed to the vast majority of chemicals, biologics, or food ingredients.
This inertness is critical. It guarantees that the gasket will not alter the product's chemical composition, taste, color, or efficacy, preserving the integrity of the final product from batch to batch.
Certified Non-Toxicity
PTFE grades used in these applications are non-toxic and comply with stringent regulatory standards, such as those set by the U.S. Food and Drug Administration (FDA).
This certification provides documented proof that the material is safe for direct contact with food and pharmaceuticals, a non-negotiable requirement for consumer safety and legal compliance.
Optimizing for Hygienic Operations
Beyond being chemically safe, a gasket material must also support a clean, sanitary processing environment. PTFE's physical surface properties are a key advantage.
The Non-Stick Advantage
PTFE has an extremely low coefficient of friction, making its surface exceptionally non-stick. This property is vital for preventing the buildup of product residue or biofilms on the gasket.
Material that adheres to a gasket surface can become a breeding ground for bacteria and is difficult to remove. PTFE's non-stick nature simplifies cleaning protocols like Clean-in-Place (CIP) and ensures a more hygienic system.
Zero Absorption, Zero Cross-Contamination
Unlike more porous materials, PTFE is non-adsorbent. It does not soak up any of the media it comes into contact with.
This prevents flavors, active ingredients, or cleaning agents from being trapped in the gasket and released into a subsequent batch, eliminating a common cause of cross-contamination.
Performance Under Demanding Process Conditions
Food and pharmaceutical processes often involve extreme temperatures and aggressive cleaning cycles. PTFE is engineered to withstand these demanding operational realities.
Resilience to Temperature Extremes
PTFE gaskets maintain their integrity across a very wide service temperature range. They are stable during high-temperature processes and, crucially, during high-heat sterilization cycles like Steam-in-Place (SIP).
This thermal stability ensures the seal remains effective and does not degrade, even after repeated exposure to extreme heat, which is essential for maintaining a sterile environment.
Resistance to Aggressive Cleaning Agents
The non-reactive nature of PTFE also extends to its ability to resist corrosion from the caustic chemicals and acids commonly used in CIP systems.
This chemical resistance ensures the gasket has a long service life and does not break down, preventing potential seal failure and process downtime.
Understanding the Trade-offs of PTFE
While PTFE is an exceptional material, no single solution is perfect. Acknowledging its limitations is key to using it effectively.
Cold Flow (Creep)
PTFE is a softer material that can be subject to "cold flow" or "creep." Under sustained pressure and temperature, the material can slowly deform and move away from the point of highest stress.
This can cause the sealing pressure to decrease over time, potentially leading to leaks. It requires proper installation, correct bolt torque specifications, and in some cases, a periodic re-torquing schedule to maintain a reliable seal.
Lower Elasticity Compared to Elastomers
Compared to rubber-like materials (elastomers) such as EPDM or silicone, PTFE is more rigid and has less "springiness."
This means it is less forgiving of flange imperfections, such as scratches or misalignment. For older or less-than-perfect equipment, a pure PTFE gasket may not seal as effectively as a more flexible elastomer or a composite gasket that incorporates PTFE.
Making the Right Choice for Your Application
Selecting the right gasket involves matching the material's properties to your specific process goals and equipment realities.
- If your primary focus is ultimate chemical purity and resistance: Virgin PTFE is the definitive choice, especially for highly sensitive biologics or aggressive active pharmaceutical ingredients (APIs).
- If your process involves significant pressure and temperature cycles: Prioritize proper installation with torque-limiting wrenches and consider a re-torquing program to counteract PTFE's tendency to creep.
- If you are sealing on older or slightly irregular flanges: Investigate modified or filled PTFE gaskets, which offer improved creep resistance and sealing capabilities while retaining excellent chemical compatibility.
By understanding PTFE's unique profile of purity, performance, and practical limitations, you can confidently ensure the integrity and safety of your critical processes.
Summary Table:
| Key Property | Benefit for Pharma & Food Industries |
|---|---|
| Chemical Inertness | Prevents contamination, ensures product integrity |
| Non-Toxicity | Meets FDA and other regulatory standards |
| Non-Stick Surface | Reduces residue buildup, simplifies cleaning |
| Temperature Resistance | Withstands sterilization and process extremes |
| Zero Absorption | Eliminates risk of cross-contamination |
Ensure the highest standards of purity and safety in your operations with KINTEK's precision PTFE components.
As specialists in manufacturing high-quality PTFE seals, gaskets, liners, and labware, we understand the critical demands of the semiconductor, medical, laboratory, and industrial sectors. Our expertise in custom fabrication—from prototypes to high-volume orders—ensures you receive components that guarantee product integrity, regulatory compliance, and operational efficiency.
Contact us today to discuss your specific needs and discover how our PTFE solutions can enhance your process safety and reliability.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Customizable PTFE Seals Filter Holders for Versatile Applications
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
People Also Ask
- Why don't PTFE O-rings swell? Discover the Key to Unmatched Chemical Stability
- Why do expanded PTFE gaskets have excellent creep resistance? Unlock Long-Term Sealing Reliability
- What electrical properties do PTFE-coated O-rings possess? Superior Surface Insulation for Static Seals
- What are the structural differences between PTFE lined and hard seal butterfly valves? A Guide to Sealing Mechanisms
- Which type of oil seal is better for extreme temperature and chemical environments? The Definitive Guide to PTFE Seals
- What are the steps for installing a PTFE gasket? A Guide to a Leak-Free Seal
- What role does chemical exposure play in selecting PTFE packing? The Ultimate Guide to Material Compatibility
- How did rotary lip seals evolve historically? From Leather to High-Performance Systems



















